Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain - PubMed (original) (raw)
Review
Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain
Jan Kaslin et al. Philos Trans R Soc Lond B Biol Sci. 2008.
Abstract
Post-embryonic neurogenesis is a fundamental feature of the vertebrate brain. However, the level of adult neurogenesis decreases significantly with phylogeny. In the first part of this review, a comparative analysis of adult neurogenesis and its putative roles in vertebrates are discussed. Adult neurogenesis in mammals is restricted to two telencephalic constitutively active zones. On the contrary, non-mammalian vertebrates display a considerable amount of adult neurogenesis in many brain regions. The phylogenetic differences in adult neurogenesis are poorly understood. However, a common feature of vertebrates (fish, amphibians and reptiles) that display a widespread adult neurogenesis is the substantial post-embryonic brain growth in contrast to birds and mammals. It is probable that the adult neurogenesis in fish, frogs and reptiles is related to the coordinated growth of sensory systems and corresponding sensory brain regions. Likewise, neurons are substantially added to the olfactory bulb in smell-oriented mammals in contrast to more visually oriented primates and songbirds, where much fewer neurons are added to the olfactory bulb. The second part of this review focuses on the differences in brain plasticity and regeneration in vertebrates. Interestingly, several recent studies show that neurogenesis is suppressed in the adult mammalian brain. In mammals, neurogenesis can be induced in the constitutively neurogenic brain regions as well as ectopically in response to injury, disease or experimental manipulations. Furthermore, multipotent progenitor cells can be isolated and differentiated in vitro from several otherwise silent regions of the mammalian brain. This indicates that the potential to recruit or generate neurons in non-neurogenic brain areas is not completely lost in mammals. The level of adult neurogenesis in vertebrates correlates with the capacity to regenerate injury, for example fish and amphibians exhibit the most widespread adult neurogenesis and also the greatest capacity to regenerate central nervous system injuries. Studying these phenomena in non-mammalian vertebrates may greatly increase our understanding of the mechanisms underlying regeneration and adult neurogenesis. Understanding mechanisms that regulate endogenous proliferation and neurogenic permissiveness in the adult brain is of great significance in therapeutical approaches for brain injury and disease.
Figures
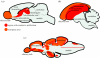
Figure 1
Parasagittal schematic overviews of the adult proliferation pattern and neurogenic regions in the brain of adult vertebrates. (a) Rodent (mouse), (b) Bird (songbird) and (c) Fish (zebrafish).
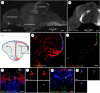
Figure 2
(a) A parasagittal overview of the proliferation zones in the brain of an adult zebrafish. The zebrafish was pulsed for 4 h with BrdU and whole mount stained. (b) A parasagittal overview of the zebrafish telencephalon stained with PSA-NCAM showing a chain of cells along the telencephalic ventricle immunopositive for the PSA-NCAM. (c) Cross-section and overview of the proliferative zones (red dots) in the everted fish telencephalon (arrow). Boxed region shows the field of view in (d) and (e). (d) A cross-section through the dorsal telencephalon of an adult zebrafish showing BrdU-labelled cells (green) along the lateral margin, as well as Hu-positive neurons (red) and S100β-positive glia (blue). The fish was pulsed for 2 days with BrdU. (e) A cross-section from a similar level to (e) showing PSA-NCAM-positive cells (red) along the lateral margin as well as S100β-positive glia (green). (f, g) Cross-section of the pretectum of an adult zebrafish showing a newly generated tyrosine hydroxylase-positive neuron. (h, i) Cross-section of the hypothalamus showing newly generated serotonin neurons (Grandel et al. 2006). DT, dorsal telencephalon; VT, ventral telencephalon; TV, tectal ventricle. (f)–(i) is reprinted with kind permission from Grandel et al. (2006), © Elsevier.
Similar articles
- Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment.
Ceci M, Mariano V, Romano N. Ceci M, et al. Rev Neurosci. 2018 Dec 19;30(1):45-66. doi: 10.1515/revneuro-2018-0020. Rev Neurosci. 2018. PMID: 30067512 Review. - Neural stem cell plasticity: recruitment of endogenous populations for regeneration.
Ferretti P. Ferretti P. Curr Neurovasc Res. 2004 Jul;1(3):215-29. doi: 10.2174/1567202043362397. Curr Neurovasc Res. 2004. PMID: 16181072 Review. - Adult neurogenesis and neuronal regeneration in the brain of teleost fish.
Zupanc GK. Zupanc GK. J Physiol Paris. 2008 Jul-Nov;102(4-6):357-73. doi: 10.1016/j.jphysparis.2008.10.007. Epub 2008 Oct 17. J Physiol Paris. 2008. PMID: 18984045 Review. - Social Cues, Adult Neurogenesis, and Reproductive Behavior.
Peretto P, Paredes RG. Peretto P, et al. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. PMID: 24830028 Free Books & Documents. Review. - Is there a relationship between adult neurogenesis and neuron generation following injury across evolution?
Ferretti P. Ferretti P. Eur J Neurosci. 2011 Sep;34(6):951-62. doi: 10.1111/j.1460-9568.2011.07833.x. Eur J Neurosci. 2011. PMID: 21929627 Review.
Cited by
- Delayed gratification in the adult brain.
Kempermann G. Kempermann G. Elife. 2020 Jul 21;9:e59786. doi: 10.7554/eLife.59786. Elife. 2020. PMID: 32690134 Free PMC article. - Neurogenesis in the Developing and Adult Brain-Similarities and Key Differences.
Götz M, Nakafuku M, Petrik D. Götz M, et al. Cold Spring Harb Perspect Biol. 2016 Jul 1;8(7):a018853. doi: 10.1101/cshperspect.a018853. Cold Spring Harb Perspect Biol. 2016. PMID: 27235475 Free PMC article. Review. - The neurogenic factor NeuroD1 is expressed in post-mitotic cells during juvenile and adult Xenopus neurogenesis and not in progenitor or radial glial cells.
D'Amico LA, Boujard D, Coumailleau P. D'Amico LA, et al. PLoS One. 2013 Jun 14;8(6):e66487. doi: 10.1371/journal.pone.0066487. Print 2013. PLoS One. 2013. PMID: 23799108 Free PMC article. - Evolution of the mammalian dentate gyrus.
Hevner RF. Hevner RF. J Comp Neurol. 2016 Feb 15;524(3):578-94. doi: 10.1002/cne.23851. Epub 2015 Jul 29. J Comp Neurol. 2016. PMID: 26179319 Free PMC article. Review. - Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish.
Zambusi A, Ninkovic J. Zambusi A, et al. World J Stem Cells. 2020 Jan 26;12(1):8-24. doi: 10.4252/wjsc.v12.i1.8. World J Stem Cells. 2020. PMID: 32110272 Free PMC article. Review.
References
- Adolf B, Chapouton P, Lam C.S, Topp S, Tannhäuser B, Strähle U, Götz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 2006;295:278–293. doi:10.1016/j.ydbio.2006.03.023 - DOI - PubMed
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969;137:433–457. doi:10.1002/cne.901370404 - DOI - PubMed
- Altman J, Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi:10.1002/cne.901240303 - DOI - PubMed
- Alvarado A.S. Regeneration in the metazoans: why does it happen? Bioessays. 2000;22:578–590. doi:10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-# - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources