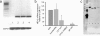Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin - PubMed (original) (raw)
Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin
Miriam Schlee et al. Infect Immun. 2007 May.
Abstract
Human beta-defensin 2 (hBD-2) is an inducible antimicrobial peptide synthesized by the epithelium to counteract bacterial adherence and invasion. Proinflammatory cytokines, as well as certain bacterial strains, have been identified as potent endogenous inducers. Recently, we have found that hBD-2 induction by probiotic Escherichia coli Nissle 1917 was mediated through NF-kappaB- and AP-1-dependent pathways. The aim of the present study was to identify the responsible bacterial factor. E. coli Nissle 1917 culture supernatant was found to be more potent than the pellet, indicating a soluble or shed factor. Chemical analysis demonstrated the factor to be heat resistant and proteinase digestible. Several E. coli Nissle 1917 deletion mutants were constructed and tested for their ability to induce hBD-2 expression in Caco-2 cells. Deletion mutants for flagellin specifically exhibited an impaired immunostimulatory capacity. Reinsertion of the flagellin gene restored the induction capacity to normal levels. Isolated flagellin from E. coli Nissle 1917 and from Salmonella enterica serovar Enteritidis induced hBD-2 mRNA significantly in contrast to the flagellin of the apathogenic E. coli strain ATCC 25922. H1 flagellin antiserum abrogated hBD-2 expression induced by flagellin as well as E. coli Nissle 1917 supernatant, confirming that flagellin is the major stimulatory factor of E. coli Nissle 1917.
Figures
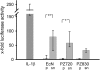
FIG. 1.
Effect of crude bacterial pellet versus supernatant on hBD-2 reporter gene activity. Transfection of Caco-2 cells with an hBD-2 promoter construct (2,338 bp) for 24 h was followed by incubation for 4.5 h with 5 ng of IL-1β or bacterial preparations/ml. The probiotic fecal E. coli isolates Nissle 1917 (EcN), PZ720, and E. coli PZ830 were adjusted to a density of 3 × 108 bacteria/ml and tested either as crude bacterial pellets (p) or corresponding supernatants (sn) in the same amount of volume. hBD-2 promoter activation was determined as a ratio between firefly and Renilla luciferase activities. The data represent the median with minimum and maximum values of three to five experiments performed in triplicate. The median was normalized to the basal luminescence of unstimulated controls, set at 1. IL-1β served as a positive stimulatory control (left column). A statistical comparison between the crude pellet and the supernatant was performed by using the Mann-Whitney test. *, P < 0.05; **, P < 0.01.
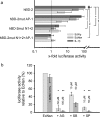
FIG. 2.
Contribution of NF-κB and AP-1 binding sites and MAP kinases for E. coli Nissle 1917 mediated hBD-2 promoter activation. (a) Various mutant promoter constructs were tested for their ability to respond to IL-1β, as well as E. coli Nissle 1917 culture supernatant (EcNsn) or crude bacterial pellet (EcN), in luciferase reporter gene assays as outlined in Fig. 1. NF-κB (positions −205 to −186 and −596 to −572) and AP-1 (positions −127 to −121) were mutated separately (hBD-2mut N1+2 or hBD-2mut AP-1) or in combination (hBD-2mut N1+2+AP-1). Maximal activity was observed with the complete promoter encompassing all known NF-κB and AP-1 sites. Sequential loss of transcription factor binding sites within the hBD-2 promoter resulted in a stepwise decrease in activation upon IL-1β, as well as pellet (EcN) and supernatant (EcNsn) treatment. The data represent the median with minimum and maximum values of duplicate samples of three to four experiments. Statistical evaluation was performed by using Kruskal-Wallis analysis with post hoc Dunn's test. *, P < 0.05; **, P < 0.01. (b) Specific MAP kinase inhibitors (diluted in DMSO) at the indicated concentrations were added 1 h prior to 4.5 h stimulation with bacterial supernatant or the corresponding supernatant, including 0.5% DMSO. Blocking of ERK1/2 by AG126 (AG) and JNK by SP600125 (SP) resulted in almost complete abrogation of E. coli Nissle 1917 supernatant-mediated hBD-2 promoter activation. Inhibition of p38 MAP kinase by SB203580 (SB) had the slightest effect. The data represent the median with minimum and maximum values of duplicate determinations of three experiments. Each inhibitor was compared to the DMSO control by Kruskal-Wallis analysis and post hoc testing. *, P < 0.05; **, P < 0.01.
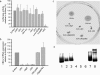
FIG. 3.
Effect of E. coli Nissle 1917 fitness factor mutants on hBD-2 expression and analysis of flagellin-mutant and complemented E. coli Nissle strains. (a) Transfected Caco-2 cells were incubated for 4.5 h with supernatants from various E. coli Nissle 1917 mutant strains (i.e., the iron siderophore yersiniabactin-negative Δ_HPI_ mutant strain; the fimbria-negative Δ_fim_, Δ_foc_, Δ_fim_Δ_foc_, and Δ_csgBA_ mutant strains; the cellulose-synthesis-negative Δ_bcs_ mutant strain; the microcin-negative Δ_mcmDAB_ mutant strain; the capsule-negative Δ_K5_ mutant strain; the plasmid-negative Δ_c_ mutant strain; and the flagellin filament protein-negative Δ_fliC_ mutant strain). hBD-2-inducing activity was determined by the luciferase reporter gene assay. Only the supernatant of the flagellin mutant (EcNsn Δ_fliC_) showed a decrease in activation ability. The data are means ± the SD of three independent experiments assayed in duplicate. (b) Induction of hBD-2 mRNA expression by flagellin-deficient E. coli Nissle 1917. Stimulation of Caco-2 cells with supernatants of different flagellin mutants (i.e., the Δ_fliC_, Δ_flgE_, and Δ_fliA_ mutant strains) for 6 h resulted in a similar decrease in hBD-2 mRNA expression. Reinsertion of the gene into the corresponding E. coli Nissle 1917 mutant [Δ_fliC_pDB2 and Δ_flgE_pDB3] completely restored mRNA induction by these strains compared to that induced by the supernatant from wild-type E. coli Nissle 1917 (EcNsn). Gene expression was analyzed by real-time PCR. The data represent means ± the SD of four experiments in duplicate. (c) Characterization of motility by swarming in semisolid agar. Cultures of the wild-type E. coli Nissle 1917 and the flagellar mutants and their complements were plated into wells punched into 0.3% LB agar plates and incubated. Visually determined swarming was normal in the case of the DH5α and wild-type E. coli Nissle 1917 (EcN) strains. All flagellin mutants (i.e., the Δ_flgE_, Δ_fliC_, and Δ_fliA_ mutant strains) showed an abolished swarming. The complemented mutants displayed an intermediate swarming [Δ_fliC_pDB2 and Δ_flgE_pDB3]. (d) Flagellin expression assessed by Western blot analysis. Heat-killed overnight bacterial cultures were subjected to SDS-PAGE electrophoresis, and flagellin expression was analyzed with H7 flagellin antiserum. Lanes: 1 and 7, EcN (wild type); 2, EcNΔ_flgE_(pDB3) (complemented Δ_flgE_ mutant); 3, EcNΔ_fliC_(pDB2) (complemented Δ_fliC_ mutant); 4, EcNΔ_flgE_; 5, EcNΔ_fliC_; 6, EcNΔ_fliA_; 8, E. coli W536. Flagellin mutants (i.e., the Δ_flgE_, Δ_fliC_, and Δ_fliA_ mutant strains) lacked the specific bands of flagellin, whereas complemented mutants (Δ_fliC_pDB2 and Δ_flgE_pDB3 mutants) displayed immunoreactivity.
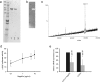
FIG. 4.
Analysis of isolated E. coli Nissle flagellin. (a) Coomassie blue staining of flagellin isolated from E. coli Nissle 1917 pellets. SDS-PAGE analysis of the purified flagellin preparation by Coomassie brilliant blue. The relevant positions of the molecular mass standard (SeeBlue Plus 2; Invitrogen) are marked on the left. Lanes 1 and 2, flagellin isolate (1 and 0.5 μg/μl); lane 3, blank. (b) Western blot of flagellin isolated from the E. coli Nissle 1917 culture supernatant. (c) Mass spectrometric analysis of isolated E. coli Nissle 1917 flagellin. The sample was diluted 1:10 in 50% acetonitrile containing 0.02% formic acid and analyzed by electrospray mass spectrometry. The determined size was 60.81 kDa. (d) Dose response of hBD-2 promoter activity by E. coli Nissle 1917 flagellin stimulation. Caco-2 cells were stimulated for 4.5 h with different concentrations of isolated flagellin. The hBD-2-inducing activity was determined by using a luciferase reporter gene assay. The relative luciferase activity is shown with the unstimulated activity of the promoter set at 1. The data are means ± the SD of three independent experiments in duplicate. (e) Effect of anti-H1 flagellin antiserum on activity of flagellin and E. coli Nissle 1917 supernatant. Isolated flagellin (0.1 μg) or E. coli Nissle 1917 supernatant (□) were mixed with H1-flagellin antiserum (1:100) in FCS-antibiotic-free DMEM and added to Caco-2 cells. The untreated flagellin and supernatant were used as a reference (▪). After 6 h of exposure, hBD-2 mRNA expression was determined by real-time PCR. Treatment of isolated flagellin or E. coli Nissle 1917 supernatant with anti-H1 flagellin antiserum reduced hBD-2 mRNA expression substantially. The data are means ± the SD of three experiments in duplicate.
FIG. 5.
Flagellin isolates induce hBD-2 mRNA expression. (a) Qualitative hBD-2 mRNA expression induced by E. coli Nissle 1917 flagellin. The results of agarose gel electrophoresis (2%) of RT-PCR products from Caco-2 cells incubated for 6 h with medium (lane 1), E. coli Nissle 1917 supernatant (lane 2), E. coli Nissle 1917 flagellin (lane 3), or serovar Enteritidis flagellin (lane 4) are shown. hBD-2 fragments are given in the upper panel, GAPDH controls of each sample are shown in the bottom panel (molecular mass marker, 100 bp). The product sizes were as follows: hBD-2, 172 bp; and GAPDH, 360 bp. (b) Quantitative hBD-2 mRNA expression induced by flagellins from different bacterial strains. Caco-2 cells were stimulated for 6 h with flagellin isolates from E. coli Nissle 1917, S. enterica serovar Enteritidis, CFT073Δ_hly, E. coli_ ATCC 25922, and E. coli JM109. hBD-2 mRNA expression was assayed by RT-PCR. The data are means ± the SD of three experiments in duplicate. (c) Western blot of flagellins from different bacterial strains. Flagellins from E. coli Nissle 1917 (lane 1), serovar Enteritidis (lane 2), CFT073Δ_hly_ (lane 3), and E. coli ATCC 25922 (lane 4) were separated by electrophoresis on SDS-PAGE gels and incubated with H1 flagellin antiserum overnight.
Similar articles
- NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium.
Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K, Schröder JM, Stange EF. Wehkamp J, et al. Infect Immun. 2004 Oct;72(10):5750-8. doi: 10.1128/IAI.72.10.5750-5758.2004. Infect Immun. 2004. PMID: 15385474 Free PMC article. - Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells.
Ogushi K, Wada A, Niidome T, Mori N, Oishi K, Nagatake T, Takahashi A, Asakura H, Makino S, Hojo H, Nakahara Y, Ohsaki M, Hatakeyama T, Aoyagi H, Kurazono H, Moss J, Hirayama T. Ogushi K, et al. J Biol Chem. 2001 Aug 10;276(32):30521-6. doi: 10.1074/jbc.M011618200. Epub 2001 May 31. J Biol Chem. 2001. PMID: 11387317 - Construction of recombinant E. coli Nissle 1917 (EcN) strains for the expression and secretion of defensins.
Seo EJ, Weibel S, Wehkamp J, Oelschlaeger TA. Seo EJ, et al. Int J Med Microbiol. 2012 Nov;302(6):276-87. doi: 10.1016/j.ijmm.2012.05.002. Epub 2012 Jun 28. Int J Med Microbiol. 2012. PMID: 22748509 - The host and the flora.
Nuding S, Antoni L, Stange EF. Nuding S, et al. Dig Dis. 2013;31(3-4):286-92. doi: 10.1159/000354680. Epub 2013 Nov 14. Dig Dis. 2013. PMID: 24246976 Review. - Genetic engineering of probiotic Escherichia coli Nissle 1917 for clinical application.
Ou B, Yang Y, Tham WL, Chen L, Guo J, Zhu G. Ou B, et al. Appl Microbiol Biotechnol. 2016 Oct;100(20):8693-9. doi: 10.1007/s00253-016-7829-5. Epub 2016 Sep 17. Appl Microbiol Biotechnol. 2016. PMID: 27640192 Review.
Cited by
- Flagellin Induces β-Defensin 2 in Human Colonic Ex vivo Infection with Enterohemorrhagic Escherichia coli.
Lewis SB, Prior A, Ellis SJ, Cook V, Chan SS, Gelson W, Schüller S. Lewis SB, et al. Front Cell Infect Microbiol. 2016 Jun 21;6:68. doi: 10.3389/fcimb.2016.00068. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27446815 Free PMC article. - Escherichia coli Nissle 1917 bacterial ghosts retain crucial surface properties and express chlamydial antigen: an imaging study of a delivery system for the ocular surface.
Montanaro J, Inic-Kanada A, Ladurner A, Stein E, Belij S, Bintner N, Schlacher S, Schuerer N, Mayr UB, Lubitz W, Leisch N, Barisani-Asenbauer T. Montanaro J, et al. Drug Des Devel Ther. 2015 Jul 21;9:3741-54. doi: 10.2147/DDDT.S84370. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26229437 Free PMC article. - No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens.
Sassone-Corsi M, Raffatellu M. Sassone-Corsi M, et al. J Immunol. 2015 May 1;194(9):4081-7. doi: 10.4049/jimmunol.1403169. J Immunol. 2015. PMID: 25888704 Free PMC article. Review. - Molecular and Cellular Mechanisms Influenced by Postbiotics.
Jastrząb R, Graczyk D, Siedlecki P. Jastrząb R, et al. Int J Mol Sci. 2021 Dec 15;22(24):13475. doi: 10.3390/ijms222413475. Int J Mol Sci. 2021. PMID: 34948270 Free PMC article. Review. - Dietary Nutrients Mediate Intestinal Host Defense Peptide Expression.
Wu J, Ma N, Johnston LJ, Ma X. Wu J, et al. Adv Nutr. 2020 Jan 1;11(1):92-102. doi: 10.1093/advances/nmz057. Adv Nutr. 2020. PMID: 31204774 Free PMC article. Review.
References
- Altenhoefer, A., S. Oswald, U. Sonnenborn, C. Enders, J. Schulze, J. Hacker, and T. A. Oelschlaeger. 2004. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med. Microbiol. 40:223-229. - PubMed
- Beatson, S. A., T. Minamino, and M. J. Pallen. 2006. Variation in bacterial flagellins: from sequence to structure. Trends Microbiol. 14:151-155. - PubMed
- Bengmark, S. 2001. Pre-, pro-, and synbiotics. Curr. Opin. Clin. Nutr. Metab. Care 4:571-579. - PubMed
- Boudeau, J., A. L. Glasser, S. Julien, J. F. Colombel, and A. Darfeuille-Michaud. 2003. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn's disease. Aliment. Pharmacol. Ther. 18:45-56. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources