Virstatin inhibits dimerization of the transcriptional activator ToxT - PubMed (original) (raw)
Virstatin inhibits dimerization of the transcriptional activator ToxT
Elizabeth A Shakhnovich et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The development of antimicrobials is critical in this time of increasing antibiotic resistance of most clinically relevant bacteria. To date, all current antibiotics focus on inhibiting crucial enzymatic activities of their protein targets (i.e., trimethoprim for dihydrofolate reductase), thus disrupting in vitro essential gene functions. In contrast, we have previously reported the identification of virstatin, a small molecule that inhibits virulence regulation in Vibrio cholerae, thereby preventing intestinal colonization in an infant mouse model for cholera. Virstatin prevents expression of the two major V. cholerae virulence factors, cholera toxin (CT) and the toxin coregulated pilus, by inhibiting the virulence transcriptional activator ToxT. It has previously been described that the N-terminal domain of ToxT has the ability to form homodimers. We now demonstrate that virstatin inhibits ToxT dimerization, thus demonstrating that it further falls into a unique class of inhibitors that works by disrupting protein-protein interactions, particularly homodimerization. Using virstatin, truncation mutants of ToxT, and a virstatin-resistant mutant, we show that dimerization is required for ToxT activation of the ctx promoter. In contrast, ToxT dimerization does not appear to be required at all of the other ToxT-regulated promoters, suggesting multiple mechanisms may exist for its transcriptional activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
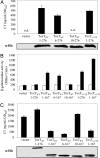
The N-terminal domain of ToxT dimerizes. (A) (Upper) Activity of ToxT at the ctx promoter when it is expressed from a plasmid in O395Δ_toxT_ as measured by CT ELISA. ToxTWT6 (aa6–276) is active, whereas ToxTWT10 (aa10–267) does not complement the toxT deletion. Full-length ToxT is 276 aa. (Lower) Western blot with α-His demonstrates that all portions of ToxT were expressed to equal levels. (B) Portions of, but not full-length, ToxT drive a protein–protein interaction in a bacterial two-hybrid system in E. coli when induced with IPTG as measured by β-galactosidase assay. Truncation of the first five amino acids allows lacZ transcription, whereas truncation of the first nine abolishes activity. Full-length and N-terminal portion of ToxTL113P point mutant (2) are able to dimerize in this system. Vector control is a transformant containing empty pACTrAp-Zif and pBRGpω plasmids. White, no IPTG; black, 10 μM IPTG. (C) (Upper) Only N-terminal portions of ToxT that can induce a protein–protein interaction can exert a dominant negative effect on CT production. ToxT truncations were expressed from a plasmid in wild-type O395. (Lower) Western blot with α-His demonstrates that all N-terminal portions were expressed to equal levels.
Fig. 2.
(A) Virstatin inhibits ToxT dimerization in bacterial two-hybrid system in E. coli. The addition of increasing concentrations of virstatin inhibited the protein–protein interaction of the ToxT N terminus with itself as measured by β-galactosidase assay. Point mutant ToxTL113P was more resistant to virstatin. Activity is presented as a percentage of reporter activity above background in the presence of virstatin compared with no virstatin. Solid line, ToxT; dashed line, ToxTL113P. (B) Chemical structure of virstatin base with the R group labeled in bold. (C) Inhibition of ToxT dimerization in the bacterial two-hybrid system and CT production in V. cholerae by analogs of virstatin is correlated. Each compound is designated by its R group and inhibition is graphed as the percentage of control activity. Virstatin is the first compound listed. White, β-galactosidase production; black, CT.
Fig. 3.
Virstatin favors ToxT monomers. (A) MBP–ToxT–His6 fusion construct. (B) The MBP–ToxT fusions complement a toxT deletion in O395 as well as wild-type ToxT. MBP–ToxTWT is inhibited by virstatin, whereas MBP–ToxTL113P is resistant to virstatin. No virstatin, black; virstatin, white. (C) (Upper) ToxTWT and ToxTL113P amounts in the absence and presence of virstatin in FPLC fractions are quantified by ELISA using Ni+2-coated plates and an anti-MBP antibody (molecular mass standards are indicated with arrows above the graph). (Lower) Western blot analysis of each of the fractions was performed by using an α-MBP antibody, demonstrating that monomeric ToxTWT can be isolated only in the presence of virstatin (bands shown are all 74 kDa).
Fig. 4.
ToxT function varies at different promoters. (A) Virstatin inhibited activity of ToxT at the ctxAB, tcpA, acfD, and tagA promoters to a greater extent than at the acfA, aldA, and tcpI promoters. Activity at each of the promoters was assayed by measuring β-galactosidase activity by using a lacZ transcriptional reporter in O395Δ_lacZ_. Data are presented as the percentage of activity in the presence of 100 μM virstatin compared with control activity. (B) Map of ToxT-binding sites at acfA and acfD promoters.
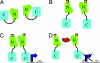
Fig. 5.
Model of ToxT activation of the ctx promoter and inhibition with virstatin. (A) The C-terminal domain of ToxT blocks the homodimerization site on the N-terminal domain in the absence of a hypothetical small molecule activator. (B) In V. cholerae, this putative activator (cube) binds to either the N- or C- terminal domains, resulting in a conformational change in ToxT, which exposes the dimerization site. (C) Dimerization of ToxT occurs, allowing binding and transcriptional activation of the ctx promoter. (D) Virstatin (red star) prevents dimerization of the N-terminal domains and transcription at the ctx promoter.
Similar articles
- Molecular mechanisms of virstatin resistance by non-O1/non-O139 strains of Vibrio cholerae.
Shakhnovich EA, Sturtevant D, Mekalanos JJ. Shakhnovich EA, et al. Mol Microbiol. 2007 Dec;66(6):1331-41. doi: 10.1111/j.1365-2958.2007.05984.x. Epub 2007 Nov 6. Mol Microbiol. 2007. PMID: 17986190 - Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization.
Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Hung DT, et al. Science. 2005 Oct 28;310(5748):670-4. doi: 10.1126/science.1116739. Epub 2005 Oct 13. Science. 2005. PMID: 16223984 - N-terminal residues of the Vibrio cholerae virulence regulatory protein ToxT involved in dimerization and modulation by fatty acids.
Childers BM, Cao X, Weber GG, Demeler B, Hart PJ, Klose KE. Childers BM, et al. J Biol Chem. 2011 Aug 12;286(32):28644-55. doi: 10.1074/jbc.M111.258780. Epub 2011 Jun 14. J Biol Chem. 2011. PMID: 21673111 Free PMC article. - Mechanism for inhibition of Vibrio cholerae ToxT activity by the unsaturated fatty acid components of bile.
Plecha SC, Withey JH. Plecha SC, et al. J Bacteriol. 2015 May;197(10):1716-25. doi: 10.1128/JB.02409-14. Epub 2015 Mar 2. J Bacteriol. 2015. PMID: 25733618 Free PMC article. - Cholera: pathophysiology and emerging therapeutic targets.
Muanprasat C, Chatsudthipong V. Muanprasat C, et al. Future Med Chem. 2013 May;5(7):781-98. doi: 10.4155/fmc.13.42. Future Med Chem. 2013. PMID: 23651092 Review.
Cited by
- Exploring the mode of action of inhibitors targeting the PhoP response regulator of Salmonella enterica through comprehensive pharmacophore approaches.
Tsai KC, Hung PP, Cheng CF, Chen C, Tseng TS. Tsai KC, et al. RSC Adv. 2019 Mar 21;9(16):9308-9312. doi: 10.1039/c9ra00620f. eCollection 2019 Mar 15. RSC Adv. 2019. PMID: 35517705 Free PMC article. - Small-molecule inhibitor of HlyU attenuates virulence of Vibrio species.
Lee ZW, Kim BS, Jang KK, Bang YJ, Kim S, Ha NC, Jung YH, Lee HJ, Han HJ, Kim JS, Kim J, Sahu PK, Jeong LS, Kim MH, Choi SH. Lee ZW, et al. Sci Rep. 2019 Mar 13;9(1):4346. doi: 10.1038/s41598-019-39554-y. Sci Rep. 2019. PMID: 30867441 Free PMC article. - Role of mgrA and sarA in methicillin-resistant Staphylococcus aureus autolysis and resistance to cell wall-active antibiotics.
Trotonda MP, Xiong YQ, Memmi G, Bayer AS, Cheung AL. Trotonda MP, et al. J Infect Dis. 2009 Jan 15;199(2):209-18. doi: 10.1086/595740. J Infect Dis. 2009. PMID: 19072553 Free PMC article. - HilD, HilC, and RtsA Form Homodimers and Heterodimers To Regulate Expression of the Salmonella Pathogenicity Island I Type III Secretion System.
Narm KE, Kalafatis M, Slauch JM. Narm KE, et al. J Bacteriol. 2020 Apr 9;202(9):e00012-20. doi: 10.1128/JB.00012-20. Print 2020 Apr 9. J Bacteriol. 2020. PMID: 32041797 Free PMC article. - Bicarbonate increases binding affinity of Vibrio cholerae ToxT to virulence gene promoters.
Thomson JJ, Withey JH. Thomson JJ, et al. J Bacteriol. 2014 Nov;196(22):3872-80. doi: 10.1128/JB.01824-14. Epub 2014 Sep 2. J Bacteriol. 2014. PMID: 25182489 Free PMC article.
References
- Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Science. 2005;310:670–674. - PubMed
- Panchal RG, Hermone AR, Nguyen TL, Wong TY, Schwarzenbacher R, Schmidt J, Lane D, McGrath C, Turk BE, Burnett J, et al. Nat Struct Mol Biol. 2004;11:67–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AI060708/AI/NIAID NIH HHS/United States
- R01 AI026289/AI/NIAID NIH HHS/United States
- AI26289/AI/NIAID NIH HHS/United States
- K08 AI060708-01/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases