Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice - PubMed (original) (raw)
Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice
Brian M Wilson et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Fragile X syndrome is a common heritable form of mental retardation in humans. Recent neuroanatomical studies indicate an apparent immature appearance of neurons in fragile X syndrome patients and fragile X mental retardation protein (FMRP)-knockout mice, an animal model of this condition. In this work, we investigated possible alterations in synaptic plasticity in the neocortex of FMRP-knockout mice. Extracellular field potentials were recorded from the deep-layer visual neocortex. Long-term potentiation (LTP) was severely attenuated in brain slices from knockout mice relative to that observed in slices from wild-type mice. Considering that neocortical LTP can involve both NMDA receptor-dependent and -independent mechanisms, we attempted to distinguish the nature of LTP attenuated in the knockout condition. In slices from wild-type mice, LTP was partially attenuated by the NMDA receptor antagonist 3-[(+/-)-2-carboxypiperazin-4-yl]-propyl-1-phosphate (CPP); however, the general metabotropic glutamate receptor (mGluR) antagonist alpha-methyl-4-carboxyphenylglycine (MCPG) strongly attenuated LTP, resulting in a response indistinguishable from that observed in slices from knockout mice. The selective mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) attenuated LTP to a similar degree as did MCPG in wild-type slices, but MPEP did not alter the reduced potentiation in knockout slices. Our results suggest that LTP in layer V visual neocortex depends primarily on mGluR5 activation. Our data also indicate that mGluR5-mediated synaptic plasticity is absent in the neocortex of FMRP-knockout mice. Such an alteration may contribute to the cognitive and learning deficits exhibited in these mice as well as in fragile X syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
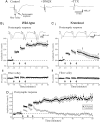
Long-term potentiation is absent in FMR1 knockout mice. (A) Representative field potential recordings from layer V of visual cortex in response to single white-matter stimulus. Each trace is an average of six consecutive traces. The response consisted of two negative peaks; the second peak is the postsynaptic response because of its sensitivity to the AMPA receptor blocker DNQX (20 μM). The initial negative peak is the presynaptic fiber volley because of its attenuation by the sodium channel blocker tetrodotoxin (TTX; 0.5 μM). (Bi) Population data from wild-type mice illustrating a stable facilitation of the postsynaptic response after tetanic stimulation. Traces are representative responses from a single slice at three different times: 1, before tetanic stimulation; 2, 5 min after LTP induction protocol; and 3, 20 min after induction protocol. (Bii) Population data for the fiber volley illustrates that the volley remains stable over the time course of the experiment. (Ci) Population data from FMRP-knockout mice illustrate a short-lasting facilitation of the postsynaptic response that returns to near baseline within 20 min after tetanic stimulation. Traces above the graph are at the same time intervals as in Bi. (Cii) Population data for the presynaptic fiber volley illustrate a stable response over the time course of the experiment. (D) Population data comparison between wild-type (■) and knockout (●) mice illustrates that the disparity in LTP is maintained up to 60 min after tetanic stimulation.
Fig. 2.
Attenuation of neocortical LTP by gluatamatergic antagonists. (A) Population data for recordings obtained from slices of wild-type mice in control conditions. (B) Population data for recordings obtained from slices of wild-type mice in the presence of the NMDA receptor antagonist CPP (20 μM). After tetanic stimulation, there was a partial reduction in the facilitation of the postsynaptic response compared with control condition. (C) In a different set of slices from wild-type animals, the LTP protocol was presented in the presence of the mGluR antagonist MCPG (500 μM). A brief facilitation of the postsynaptic response was observed after tetanic stimulation, but the response returned to near baseline levels within 20 min after stimulation. (D) In the presence of both 500 μM MCPG and 20 μM CPP, tetanic stimulation produced very little facilitation immediately after tetanic stimulation as well as no facilitation at 20 min after it.
Fig. 3.
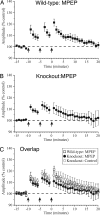
mGluR5 antagonist attenuates LTP in neocortical slices. (A) Population data from slices of wild-type mice in the presence of the selective mGluR5 antagonist MPEP (50 μM). Tetanic stimulation produces a brief facilitation in the postsynaptic response that returns to near baseline levels within 20 min after tetanic stimulation. (B) Population data from slices of FMRP-knockout mice in the presence of 50 μM MPEP. Tetanic stimulation produces a brief facilitation in the postsynaptic response that returns to near baseline levels within 20 min after tetanic stimulation. (C) Overlap of population data from slices of wild-type mice in the presence of MPEP (□), knockout mice in the presence of MPEP (●), and knockout mice in control conditions (○).
Similar articles
- Early postnatal plasticity in neocortex of Fmr1 knockout mice.
Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Desai NS, et al. J Neurophysiol. 2006 Oct;96(4):1734-45. doi: 10.1152/jn.00221.2006. Epub 2006 Jul 5. J Neurophysiol. 2006. PMID: 16823030 - Reversal of activity-mediated spine dynamics and learning impairment in a mouse model of Fragile X syndrome.
Boda B, Mendez P, Boury-Jamot B, Magara F, Muller D. Boda B, et al. Eur J Neurosci. 2014 Apr;39(7):1130-7. doi: 10.1111/ejn.12488. Eur J Neurosci. 2014. PMID: 24712992 - Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors.
Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Nakamoto M, et al. Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15537-42. doi: 10.1073/pnas.0707484104. Epub 2007 Sep 19. Proc Natl Acad Sci U S A. 2007. PMID: 17881561 Free PMC article. - BDNF in fragile X syndrome.
Castrén ML, Castrén E. Castrén ML, et al. Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review. - The role of metabotropic glutamate receptor 5 in learning and memory processes.
Simonyi A, Schachtman TR, Christoffersen GR. Simonyi A, et al. Drug News Perspect. 2005 Jul-Aug;18(6):353-61. doi: 10.1358/dnp.2005.18.6.927927. Drug News Perspect. 2005. PMID: 16247513 Review.
Cited by
- Activity-dependent modulation of neural circuit synaptic connectivity.
Tessier CR, Broadie K. Tessier CR, et al. Front Mol Neurosci. 2009 Jul 30;2:8. doi: 10.3389/neuro.02.008.2009. eCollection 2009. Front Mol Neurosci. 2009. PMID: 19668708 Free PMC article. - Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses.
Popkirov SG, Manahan-Vaughan D. Popkirov SG, et al. Cereb Cortex. 2011 Mar;21(3):501-9. doi: 10.1093/cercor/bhq093. Epub 2010 Jun 4. Cereb Cortex. 2011. PMID: 20525770 Free PMC article. - Neuropeptide Release is Impaired in a Mouse Model of Fragile X Mental Retardation Syndrome.
Annangudi SP, Luszpak AE, Kim SH, Ren S, Hatcher NG, Weiler IJ, Thornley KT, Kile BM, Wightman RM, Greenough WT, Sweedler JV. Annangudi SP, et al. ACS Chem Neurosci. 2010 Jan 8;1(4):306-314. doi: 10.1021/cn900036x. ACS Chem Neurosci. 2010. PMID: 20495672 Free PMC article. - The fragile X mental retardation protein in circadian rhythmicity and memory consolidation.
Gatto CL, Broadie K. Gatto CL, et al. Mol Neurobiol. 2009 Apr;39(2):107-29. doi: 10.1007/s12035-009-8057-0. Epub 2009 Feb 12. Mol Neurobiol. 2009. PMID: 19214804 Free PMC article. Review. - Homeostatic responses fail to correct defective amygdala inhibitory circuit maturation in fragile X syndrome.
Vislay RL, Martin BS, Olmos-Serrano JL, Kratovac S, Nelson DL, Corbin JG, Huntsman MM. Vislay RL, et al. J Neurosci. 2013 Apr 24;33(17):7548-58. doi: 10.1523/JNEUROSCI.2764-12.2013. J Neurosci. 2013. PMID: 23616559 Free PMC article.
References
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, Shubek L, Holmgreen P, Yeargin-Allsopp M, Boyle C, et al. Am J Med Genet. 2002;110:226–233. - PubMed
- Berry-Kravis E. Dev Med Child Neurol. 2002;44:724–728. - PubMed
- Hagerman RJ, Staley LW, O'Conner R, Lugenbeel K, Nelson D, McLean SD, Taylor A. Pediatrics. 1996;97:122–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY014024/EY/NEI NIH HHS/United States
- R01 EY014024/EY/NEI NIH HHS/United States
- R01 EY014024-01A1/EY/NEI NIH HHS/United States
- R01 EY014024-04/EY/NEI NIH HHS/United States
- R01 EY014024-02/EY/NEI NIH HHS/United States
- R01 EY014024-03/EY/NEI NIH HHS/United States
- R56 EY014024/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases