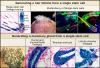
Epithelial stem cells: turning over new leaves - PubMed (original) (raw)
Review
Epithelial stem cells: turning over new leaves
Cédric Blanpain et al. Cell. 2007.
Abstract
Most epithelial tissues self-renew throughout adult life due to the presence of multipotent stem cells and/or unipotent progenitor cells. Epithelial stem cells are specified during development and are controlled by epithelial-mesenchymal interactions. Despite morphological and functional differences among epithelia, common signaling pathways appear to control epithelial stem cell maintenance, activation, lineage determination, and differentiation. Additionally, deregulation of these pathways can lead to human disorders including cancer. Understanding epithelial stem cell biology has major clinical implications for the diagnosis, prevention, and treatment of human diseases, as well as for regenerative medicine.
Figures
Figure 1. Stem Cell Location in Different Epithelial Tissues
The intestinal crypt. The putative stem cells of the intestine (red) reside in a narrow band near the base of the crypt. These stem cells are thought to move downward to differentiate into Paneth cells (blue) or upward to generate proliferative transiently amplifying (TA) cells (orange). The intestinal TA cells differentiate upward into three lineages, enteroendocrine cells, goblet cells, and enterocytes, to form the villus. The corneal limbus. The cornea (blue) is a stratified epithelium that is flanked by the limbus (red) and the conjunctiva (purple). Corneal stem cells are thought to reside in the limbus region. The hair-follicle bulge. Label-retaining stem cells of the hair follicle reside below the sebaceous gland in a region known as the bulge, which is connected to the arector pili muscle. During periods of rest, bulge stem cells form the base of the follicle, which is adjacent to the specialized mesenchymal cells (dermal papillae). At the start of each hair cycle, stem cells at the bulge base become activated to form the highly proliferative new hair germ. As the germ grows, a proliferative compartment of TA cells (matrix cells) engulfs the dermal papilla at the base. These cells progress to differentiate to form seven concentric shells of discrete cell lineages, which are from outer to inner: the companion layer, the three layers of the inner root sheath, and the three layers of the hair shaft. These differentiated layers are surrounded by the outer root sheath, which extends below the bulge and is thought to contain stem cells that continue to migrate down to the follicle base during the growth phase of the hair cycle (Oshima et al., 2001). The interfollicular epidermis is a stratified epithelium with a basal layer that contains unipotent progenitor cells and TA cells. Basal cells differentiate upward to form the spinous, granular, and stratum corneum layers of the epidermis. The mammary gland terminal end bud. Stem cells of the mammary gland reside in the terminal end bud at the end of the mammary duct. The end of the terminal end bud is composed of body cells surrounded by cap cells. During pregnancy, the stem cells form a complex ductal structure containing ductal cells that end in the alveoli that produce milk components.
Figure 2. Multipotency of Hair-Follicle and Mammary Gland Stem Cells
Bulge stem cell lineage tracing in vivo. Skin sections from mice expressing a regulatable Cre-recombinase under the control of a bulge-specific (K15) enhancer element and a lacZ transgene active in all Cre-expressing cells and their progeny (reprinted by permission from MacMillan Publishers Ltd.; Morris et al., 2004). Following Cre activation, all lineages of the new hair follicle expressed LacZ, demonstrating that they arose from bulge cells. Multipotency of bulge cells. CD34+, α6 integrin+ bulge cells were purified by flow cytometry from skin of mice expressing GFP-actin under the control of the K14 promoter (Blanpain et al., 2004). Bulge cells were cultured, and clonally derived keratinocytes from a single cell were used for grafting with unlabeled mesenchymal cells. Nude mice developed tufts of hair at grafted sites (left), and sectioning revealed that the GFP+ cells contributed to the epidermis, sebaceous glands (SG), and hair follicles (reprinted from Cell with permission from Elsevier; Blanpain et al., 2004). Multipotency of mammary gland stem cells. Lin−CD29hiCD24+ myoepithelial cells were purified by flow cytometry from lacZ+ mice, and individual cells were transplanted into cleared fat pads (Shackleton et al., 2006; Stingl et al., 2006) Epithelial grafts from virgin (left and middle) or pregnant recipients (right) demonstrate the ability of single lacZ+ stem cells (blue) to form a complete mammary gland structure. Sections of mammary grafts demonstrate the contribution of LacZ+ (blue) progeny from the single mammary stem cell to the ductal (arrowheads) and myoepithelial (arrows) cell lineages, the terminal end bud in a virgin recipient, and the lobulo-alveolar epithelium in a mid-term pregnant recipient (right, arrows indicate lipid droplets) (reprinted with permission from MacMillan Publishers Ltd: Shackleton et al., 2006).
Figure 3. Wnt/β-Catenin Signaling Regulates Epithelial Stem Cells
(A) Model summarizing the functions of Wnt/β-catenin signaling in the intestine that regulate stem cell renewal and Paneth cell differentiation. β-catenin and Tcf4 appear to regulate c-Myc and EphB2/B3 during renewal, whereas these transcriptional complexes regulate Paneth cell genes during differentiation. (B) Model summarizing functions that have been suggested for Wnt signaling in stem cell maintenance, activation, and hair shaft differentiation in the follicle. Tcf3/4 regulate genes implicated in stem cell quiescence and differentiation, whereas β-catenin and Tcf/Lef complexes regulate genes likely involved in stem cell activation and migration. β-catenin and Lef1 complexes in precortical cells regulate expression of hair shaft genes. (C) Model summarizing putative functions for Wnt signaling in the mammary gland in cancer development and alveolar differentiation. In combination with Tcf4 and Lef1, β-catenin regulates genes involved in alveolar differentiation. (D) General model depicting these different outcomes: stem cell self-renewal, activation, differentiation, or tumorigenesis, depending upon the level of effective Wnt signaling (that is, β-catenin/Lef/Tcf activity) that an epithelial stem cell receives.
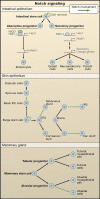
Figure 4. Notch Signaling Regulates Lineages Determination of Epithelial Stem Cells
In canonical Notch signaling, upon binding to Notch ligands, Notch receptors are cleaved and the intracellular domain translocates to the nucleus to act in concert with an RBP-J transcription factor to regulate gene expression. The schematics illustrate the putative roles of Notch signaling in three different epithelial tissues. (Top) A role for Notch in intestinal stem cell self-renewal and differentiation. Notch/RBP-J restricts the fate of secretory lineages in the intestine (Goblet, Paneth, and enteroendocrine cells). In the absorptive progenitors, Notch activity leads to the expression of Hes1 and subsequent repression of Math1, leading to enterocyte differentiation. In the absence of Notch signaling, Math1 expression is not repressed and allows secretory progenitors to differentiate into goblet, neuroendrocrine, and Paneth cells. (Middle) A role for Notch in stem cell maintenance and differentiation in the epidermis and hair follicle. Notch promotes spinous cell fate in the interfollicular epidermis (IFE) and regulates differentiation of the sebaceous gland, the hair shaft, and the inner root sheath cells of the hair follicle. (Bottom) A role for Notch signaling in alveolar differentiation in the mammary gland. Mammary stem cells form alveolar and ductal progenitors that each form a myoepithelial cell in addition to tubular and alveolar cells, respectively. Notch signaling regulates the differentiation of alveolar progenitors into alveolar cells.
Figure 5. Regenerative Potential of Epidermal and Corneal Epithelium
Top, from left to right: Colonies of human keratinocytes grown on a fibrin matrix can be used to form an epithelial sheet. These sheets were used to treat a patient with burns on over 95% of his body (top image). After 3.5 years, the transplantation has completely healed the patient's skin (bottom image). Histologically, the long-term transplant was normal and contained all of the normal epidermal layers and architecture (reprinted with permission from Lippincott, Williams, and Wilkins; Ronfard et al., 2000). Bottom, from left to right: Long-term potential of the cornea from patient with alkali burns to the cornea causing stromal scarring and neovascularization (Pellegrini, et al., 1997). Cultures of limbal stem cells isolated from a biopsy of the contralateral eye were transplanted and were able to repair the damaged cornea and produce a healthy cornea, allowing 100% recovery of visual acuity. A biopsy from the regenerated cornea demonstrated that the tissue organization was normal (Courtesy of Drs. Graziella Pellegrini, Paolo Rama, and Michelle DeLuca).
Similar articles
- Lineage enforcement by inductive mesenchyme on adult epithelial stem cells across developmental germ layers.
Taylor RA, Wang H, Wilkinson SE, Richards MG, Britt KL, Vaillant F, Lindeman GJ, Visvader JE, Cunha GR, St John J, Risbridger GP. Taylor RA, et al. Stem Cells. 2009 Dec;27(12):3032-42. doi: 10.1002/stem.244. Stem Cells. 2009. PMID: 19862839 - Kidney stem cells in development, regeneration and cancer.
Dziedzic K, Pleniceanu O, Dekel B. Dziedzic K, et al. Semin Cell Dev Biol. 2014 Dec;36:57-65. doi: 10.1016/j.semcdb.2014.08.003. Epub 2014 Aug 13. Semin Cell Dev Biol. 2014. PMID: 25128731 Review. - Have hair follicle stem cells shed their tranquil image?
Lin KK, Andersen B. Lin KK, et al. Cell Stem Cell. 2008 Dec 4;3(6):581-2. doi: 10.1016/j.stem.2008.11.005. Cell Stem Cell. 2008. PMID: 19041772 Review. - The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids.
Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, Berger H, Mollenkopf HJ, Mangler M, Sehouli J, Fotopoulou C, Meyer TF. Kessler M, et al. Nat Commun. 2015 Dec 8;6:8989. doi: 10.1038/ncomms9989. Nat Commun. 2015. PMID: 26643275 Free PMC article. - Heterotypic cell-cell communication regulates glandular stem cell multipotency.
Centonze A, Lin S, Tika E, Sifrim A, Fioramonti M, Malfait M, Song Y, Wuidart A, Van Herck J, Dannau A, Bouvencourt G, Dubois C, Dedoncker N, Sahay A, de Maertelaer V, Siebel CW, Van Keymeulen A, Voet T, Blanpain C. Centonze A, et al. Nature. 2020 Aug;584(7822):608-613. doi: 10.1038/s41586-020-2632-y. Epub 2020 Aug 26. Nature. 2020. PMID: 32848220 Free PMC article.
Cited by
- Stem cell therapy: a novel & futuristic treatment modality for disaster injuries.
Gurudutta GU, Satija NK, Singh VK, Verma YK, Gupta P, Tripathi RP. Gurudutta GU, et al. Indian J Med Res. 2012;135(1):15-25. doi: 10.4103/0971-5916.93419. Indian J Med Res. 2012. PMID: 22382178 Free PMC article. Review. - Very small embryonic/epiblast-like stem cells (VSELs) and their potential role in aging and organ rejuvenation--an update and comparison to other primitive small stem cells isolated from adult tissues.
Ratajczak MZ, Shin DM, Liu R, Mierzejewska K, Ratajczak J, Kucia M, Zuba-Surma EK. Ratajczak MZ, et al. Aging (Albany NY). 2012 Apr;4(4):235-46. doi: 10.18632/aging.100449. Aging (Albany NY). 2012. PMID: 22498452 Free PMC article. Review. - Safety and therapeutic potential of human bone marrow-derived mesenchymal stromal cells in regenerative medicine.
Aithal AP, Bairy LK, Seetharam RN. Aithal AP, et al. Stem Cell Investig. 2021 May 11;8:10. doi: 10.21037/sci-2020-036. eCollection 2021. Stem Cell Investig. 2021. PMID: 34124233 Free PMC article. Review. - Regeneration of multilineage skin epithelia by differentiated keratinocytes.
Mannik J, Alzayady K, Ghazizadeh S. Mannik J, et al. J Invest Dermatol. 2010 Feb;130(2):388-97. doi: 10.1038/jid.2009.244. Epub 2009 Aug 13. J Invest Dermatol. 2010. PMID: 19675579 Free PMC article. - Role of FcγRIII in the nasal cavity of BALB/c mice in the primary amebic meningoencephalitis protection model.
Rojas-Ortega DA, Rojas-Hernández S, Sánchez-Mendoza ME, Gómez-López M, Sánchez-Camacho JV, Rosales-Cruz E, Yépez MMC. Rojas-Ortega DA, et al. Parasitol Res. 2023 May;122(5):1087-1105. doi: 10.1007/s00436-023-07810-w. Epub 2023 Mar 13. Parasitol Res. 2023. PMID: 36913025 Free PMC article.
References
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. - PubMed
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2002;2:643–653. - PubMed
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources