Reversal of neurological defects in a mouse model of Rett syndrome - PubMed (original) (raw)
Reversal of neurological defects in a mouse model of Rett syndrome
Jacky Guy et al. Science. 2007.
Abstract
Rett syndrome is an autism spectrum disorder caused by mosaic expression of mutant copies of the X-linked MECP2 gene in neurons. However, neurons do not die, which suggests that this is not a neurodegenerative disorder. An important question for future therapeutic approaches to this and related disorders concerns phenotypic reversibility. Can viable but defective neurons be repaired, or is the damage done during development without normal MeCP2 irrevocable? Using a mouse model, we demonstrate robust phenotypic reversal, as activation of MeCP2 expression leads to striking loss of advanced neurological symptoms in both immature and mature adult animals.
Figures
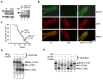
Fig. 1. Insertion of a _lox_-Stop cassette into intron 2 of the mouse Mecp2 gene creates an allele that is effectively null, but can be activated by TM treatment.
(A) Western blot analysis of MeCP2 protein (solid arrow) in brains of wildtype (wt), Stop/y and Stop/y,cre mice before and after TM. Anti-MeCP2 antibodies were from J. Pevsner (left panel) and Upstate (right panel). Internal controls are non-specific cross-reacting bands (!) and bands generated by an anti-histone H4 antibody (open arrow). (B) Detection of MeCP2 by in situ immunofluorescence in dentate gyrus of wildtype (wt), Stop/y and TM-treated Stop/y,cre mice. White scale bar = 50μm. Green DAPI-negative cells in the upper Stop panel are non-nucleate erythrocytes showing background fluorescence. The DAPI channel was changed from blue to red using Adobe Photoshop to contrast with the green MeCP2 signal. (C) Comparison of the survival of Stop/y mice with and without the _cre_-ER transgene. (D) A southern blot assay for deletion of the _Lox_-Stop cassette in brains of heterozygous Stop/+,cre females (f) aged 10 months (lane 2 and 3) that had not been exposed to TM. Restriction fragments from the Mecp2 _lox_-Stop (Stop; see male Mecp2 lox-Stop/y, lane 1), deleted Mecp2 D (Δ, see lane 4) and the wildtype (wt) alleles are indicated. (E) Southern blot assay for conversion of the Stop allele to the Mecp2 D allele (Δ) in male mouse brains after 5 daily TM injections. Lanes 2 and 5 show the wt allele.
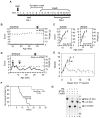
Fig. 2. Reversal of the neurological phenotype by activation of the Mecp2 gene in Stop/y,cre males.
(A) Timecourse of the Stop/y phenotype. (B, C and D Plots of the phenotypic scores (•) and weights (x) of individual wildtype (wt; B), Stop/y (Stop; C) and Mecp2 lox-Stop/y,_cre_-ER (_Stop_-cre; D) animals following TM injections (vertical arrows). See also Fig. S2. Stars (panel D) indicate when the clips shown in Movies S1 and S2 were recorded. (E) Aggregate symptom score profiles following TM injection of Stop/y,cre (•, n = 3-6, except * which was a single animal) and Stop/y (•, n = 4-5; except ## and # which are 2 and 1 data points, respectively) mice. (F) Survival profiles of TM-treated Stop/y,cre mice and control Stop/y mice. (G) Southern blot showing deletion of the lox-Stop cassette (lanes 3 and 5) following a weekly TM injection regime + booster injections.
Fig. 3. Reversal of late-onset neurological symptoms by Mecp2 gene induction in mature adult Stop/+,cre females.
(A) Time course of symptom onset. TM administration began during the bracketed period (TM3). (B, C and D) Phenotype (•) and weight (x) profiles for a Stop/+ female (B) and two Stop/+,cre females (C and D). All animals shown were subjected to either 5 daily injections or 5 weekly TM injections plus 3 booster injections (vertical arrows). Animals subject to weekly injection regimes were scored blind as part of a mixed genotype cohort. (E) Plot of average symptom scores for females with wt (⋄, n = 5-6), Stop/+ (▴, n = 6-7), and Stop,+,cre (•, n = 5-11) genotypes. Repeated measures ANOVA compared Stop/+ and Stop/+,cre female scores in weeks 11 – 16. F. Southern blot analysis of the effects of TM treatment on a cohort including 6 Stop/+,cre (lanes 5-10), 6 wt (lanes 11-16) and 6 Stop/+ (lanes 17-22) females. All three genotypes received TM. Restriction fragments derived from Mecp2 _lox_-Stop (Stop), deleted Mecp2 D (Δ) and wildtype (wt) are arrowed. Brain DNA from animals #32 and #5 shown above are in lanes 9 and 6, respectively. Lanes 1 – 4 show blots of wt male, Stop/y male, Stop/+ female, and Mecp2 D /+ female, respectively.
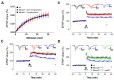
Fig. 4. A deficit in long-term potentiation (LTP) accompanies onset of symptoms in mature adult Mecp2 lox-Stop/+ heterozygous females and is reversed by Mecp2 reactivation.
(A) Stimulation-response curves in symptomatic (blue) or pre- symptomatic (red) Mecp2 +/- female mice and wt littermate controls (black; all P>0.05). (B) Measurements of LTP using a high frequency stimulation (HFS) paradigm in pre- symptomatic (n = 7 - 9), symptomatic (n = 8 - 9) Mecp2 +/- mice and wt female littermate controls (n = 7 - 8; all P<0.05). C. Measurements of LTP using thetaburst stimulation in pre-symptomatic (n = 7 - 9), symptomatic (n = 8 - 9) Mecp2 +/- and wt female littermate controls (n = 7 - 8; all P<0.05). D. HFS-induced LTP measurements in TM-treated symptomatic Stop/+ mice (n = 11; P<0.05), Stop/+,cre mice (n = 10) and wt mice (n = 9; P<0.05). Recombination data is shown in Fig. 3F. Insets in (B - D) show representative voltage traces before (1) and after (2) LTP induction. Two way repeated measures ANOVA was used to assess significance throughout.
Similar articles
- Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice.
Jugloff DG, Vandamme K, Logan R, Visanji NP, Brotchie JM, Eubanks JH. Jugloff DG, et al. Hum Mol Genet. 2008 May 15;17(10):1386-96. doi: 10.1093/hmg/ddn026. Epub 2008 Jan 25. Hum Mol Genet. 2008. PMID: 18223199 - Radically truncated MeCP2 rescues Rett syndrome-like neurological defects.
Tillotson R, Selfridge J, Koerner MV, Gadalla KKE, Guy J, De Sousa D, Hector RD, Cobb SR, Bird A. Tillotson R, et al. Nature. 2017 Oct 19;550(7676):398-401. doi: 10.1038/nature24058. Epub 2017 Oct 11. Nature. 2017. PMID: 29019980 Free PMC article. - Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes.
Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Chao HT, et al. Nature. 2010 Nov 11;468(7321):263-9. doi: 10.1038/nature09582. Nature. 2010. PMID: 21068835 Free PMC article. - Reversibility of functional deficits in experimental models of Rett syndrome.
Cobb S, Guy J, Bird A. Cobb S, et al. Biochem Soc Trans. 2010 Apr;38(2):498-506. doi: 10.1042/BST0380498. Biochem Soc Trans. 2010. PMID: 20298210 Review. - MeCP2 and Rett syndrome: reversibility and potential avenues for therapy.
Gadalla KK, Bailey ME, Cobb SR. Gadalla KK, et al. Biochem J. 2011 Oct 1;439(1):1-14. doi: 10.1042/BJ20110648. Biochem J. 2011. PMID: 21916843 Review.
Cited by
- A general approach for controlling transcription and probing epigenetic mechanisms: application to the CD4 locus.
Wan M, Kaundal R, Huang H, Zhao J, Yang X, Chaiyachati BH, Li S, Chi T. Wan M, et al. J Immunol. 2013 Jan 15;190(2):737-47. doi: 10.4049/jimmunol.1201278. J Immunol. 2013. PMID: 23293358 Free PMC article. - Rett syndrome: a complex disorder with simple roots.
Lyst MJ, Bird A. Lyst MJ, et al. Nat Rev Genet. 2015 May;16(5):261-75. doi: 10.1038/nrg3897. Epub 2015 Mar 3. Nat Rev Genet. 2015. PMID: 25732612 Review. - Influence of stimulant-induced hyperactivity on social approach in the BTBR mouse model of autism.
Silverman JL, Babineau BA, Oliver CF, Karras MN, Crawley JN. Silverman JL, et al. Neuropharmacology. 2013 May;68:210-22. doi: 10.1016/j.neuropharm.2012.07.042. Epub 2012 Aug 8. Neuropharmacology. 2013. PMID: 22968082 Free PMC article. - Interneuron dysfunction in psychiatric disorders.
Marín O. Marín O. Nat Rev Neurosci. 2012 Jan 18;13(2):107-20. doi: 10.1038/nrn3155. Nat Rev Neurosci. 2012. PMID: 22251963 Review. - The relationship of Rett syndrome and MECP2 disorders to autism.
Neul JL. Neul JL. Dialogues Clin Neurosci. 2012 Sep;14(3):253-62. doi: 10.31887/DCNS.2012.14.3/jneul. Dialogues Clin Neurosci. 2012. PMID: 23226951 Free PMC article. Review.
References
- Amir RE, et al. Nature Genet. 1999;23:185. - PubMed
- Neul JL, Zoghbi HY. Neuroscientist. 2004 Apr;10:118. - PubMed
- Armstrong D, Dunn JK, Antalffy B, Trivedi R. J Neuropathol Exp Neurol. 1995 Mar;54:195. - PubMed
- Kishi N, Macklis JD. Mol Cell Neurosci. 2004 Nov;27:306. - PubMed
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Nat Genet. 2001;27:327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials