Prefrontal cortical network activity: Opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation - PubMed (original) (raw)
Prefrontal cortical network activity: Opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation
E K Lambe et al. Neuroscience. 2007.
Abstract
The fine-tuning of network activity provides a modulating influence on how information is processed and interpreted in the brain. Here, we use brain slices of rat prefrontal cortex to study how recurrent network activity is affected by neuromodulators known to alter normal cortical function. We previously determined that glutamate spillover and stimulation of extrasynaptic N-methyl-d-aspartic acid (NMDA) receptors are required to support hallucinogen-induced cortical network activity. Since microdialysis studies suggest that psychedelic hallucinogens and dopamine D1/D5 receptor agonists have opposite effects on extracellular glutamate in prefrontal cortex, we hypothesized that these two families of psychoactive drugs would have opposite effects on cortical network activity. We found that network activity can be enhanced by 2,5-dimethoxy-4-iodoamphetamine (DOI) (a psychedelic hallucinogen that is a partial agonist of 5-HT(2A/2C) receptors) and suppressed by the selective D1/D5 agonist SKF 38393. This suppression could be mimicked by direct activation of adenylyl cyclase with forskolin or by addition of a cAMP analog. These findings are consistent with previous work showing that activation of adenylyl cyclase can upregulate neuronal glutamate transporters, thereby decreasing synaptic spillover of glutamate. Consistent with this hypothesis, a low concentration of the glutamate transporter inhibitor threo-beta-benzoylaspartic acid (TBOA) restored electrically-evoked recurrent activity in the presence of a selective D1/D5 agonist, whereas recurrent activity in the presence of a low level of the GABA(A) antagonist bicuculline was not resistant to suppression by the D1/D5 agonist. The tempering of network UP states by D1/D5 receptor activation may have implications for the proposed use of D1/D5 agonists in the treatment of schizophrenia.
Figures
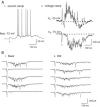
Figure 1
Recurrent network activity can be evoked electrically in brain slice of rat prefrontal cortex. (A) Current and voltage clamp recordings from a neuron under basal conditions. (1) A stimulus (10 μA; gray arrow) evokes network activity in the slice that depolarizes the neuron and increases its synaptic noise and spiking. (2) Voltage-clamping this neuron at different potentials allows the visualization of the inhibitory and excitatory aspects of the fast PSC and electrically-evoked network activity in the slice (VH = holding potential). No channel inhibitors are applied. (B) The oscillations of the basal and DOI-enhanced network activity show characteristic gamma wavelength activity (30 – 80 Hz). Single sweeps in voltage clamp (VH = −75 mV) show electrically-evoked (0.1 Hz) fast EPSCs and the excitatory component of the evoked network activity under basal conditions and following application of the psychedelic hallucinogen DOI (3 μM; 15 minutes).
Figure 2
A psychedelic hallucinogen and a D1/D5 agonist have opposite effects on recurrent network activity in brain slice of rat prefrontal cortex. (A) Average of 10 sweeps (0.1 Hz) in voltage-clamp (VH = −78 mV) showing the electrically-evoked fast EPSC and the excitatory component of evoked network activity (late EPSCs): (1) under basal conditions, (2) after application of DOI (3 μM; 5 minutes), (3) during application of SKF 38393 (100 nM; 4 minutes), and (4) after 15 minutes washout from SKF 38393. Note the delay in evoked network activity during washout of SKF 38393. (B) Bar graph shows the areas (mean ± S.E.) of the electrically-evoked fast EPSC and the late EPSC or network activity in the above conditions (N = 10 neurons). DOI significantly enhanced the area of the late EPSC, and SKF 38393 significantly suppressed the late EPSC. * denotes P < 0.01; ** denotes P < 0.00001. (C) Graph shows the time course of network activity and the evolution of the drug effects on a representative neuron. Each circle is the average of the 6 electrically-evoked network events in voltage-clamp that are stimulated during each one minute period (0.1 Hz stimulus frequency). DOI (3 μM; 5 min) and SKF 38393 (100 nM, 4 min) were applied as indicated. It must be noted that the DOI-mediated enhancement of network activity is slow to wash out and remains at a peak for hours. (D) Superimposed averaged sweeps in voltage clamp during sequential application of increasing doses of SKF 38393 (0 nM to 300 nM; 4 minute applications) for one neuron. As shown, the fast EPSC has not changed, but the network activity is dose-dependently suppressed. (D) Dose response curve from 6 neurons for the SKF 38393 suppression of DOI-enhanced network activity.
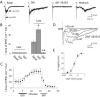
Figure 3
Evoked network activity is suppressed selectively through D1/D5 activation. (A) Averaged sweeps in voltage clamp showing electrically-evoked fast EPSC and the excitatory component of the evoked network activity (late EPSCs): (1) under basal conditions, (2) after application of DOI, (3) during application of dopamine (10 μM); (4) after washout of dopamine; (5) during pre-application of raclopride (300 nM; 10 minutes); (6) during co-application of raclopride (300 nM) and dopamine (10 μM); (7) after pre-application of SCH 23390 (300 nM; 15 minutes); and (8) during application of dopamine (10 μM). (B) Bar chart showing the percentage by which dopamine (3-10 μM) suppresses the fast and the late EPSCs under basal conditions and in the presence of the D2 antagonist, raclopride (300 nM – 1 μM; N = 4), or after application of the D1/D5 antagonist SCH23390 (300 nM; 10 minutes; N = 5). Dopamine significantly suppressed the late EPSC under basal conditions and in the presence of raclopride; * denotes P < 0.01. Except for illustration in part (A) of this figure, raclopride and SCH23390 were not used in the same slices. (C) Bar chart shows the percentage by which the respective D2 and D1/D5 agonists, quinpirole (quin; 1 μM; 10 minutes; N = 4) and SKF 38393 (SKF; 100 nM; 4 minutes; N = 10) suppress the fast and the late EPSCs under basal conditions and in the presence of antagonists. Only SKF 38393 suppressed evoked network activity or late EPSCs (* denotes P < 0.001). This suppression was blocked by pre-application of the selective D1/D5 receptor antagonists SCH 23390 (300 nM; 20 minutes; N = 6) or SCH 36199 (1 μM; 15-20 minutes; N = 5).
Figure 4
Second messengers in the G s pathway suppress evoked network activity. (A) Bar chart shows the mean ± S.E. data for the effects of forskolin (direct activator of adenylyl cyclase; 10 μM; 4 minutes; N = 4) and 8-Br-cAMP (0.5 mM; 5-10 minutes; N = 4) on the fast EPSC and evoked network activity (late EPSCs). Both treatments selectively suppressed network activity (* denotes P < 0.01). (B) Averaged sweeps in voltage clamp showing that 8-Br-cAMP (0.5 mM; 5 minutes) suppresses the evoked network activity without changing the fast EPSC.
Figure 5
SKF 38393 suppresses network activity in the presence of a low dose of the GABA-A receptor antagonist, bicuculline methiodide. (A1) Averaged traces show the electrically-evoked fast EPSC and network activity in the presence of a low dose of bicuculline (BIC; 1 μM; 3 minutes). (2) In continued presence of bicuculline (1 μM), SKF 38393 (100 nM; 5 min) suppresses network activity but does not alter the fast EPSC in this neuron. (B) The bar chart shows the mean ± S.E. for the area of the fast EPSC and the excitatory component of evoked network activity (late EPSCs) during application of bicuculline alone (1 μM; 3-5 min) and then bicuculline (1μM) co-applied with SKF 38393 (100 nM; 3-5 min). SKF 38393 dramatically reduced network activity (N = 6; paired t test; ** denotes P < 0.01). The fast EPSC was also slightly suppressed by SKF 38393 (N = 6; paired t test; * denotes P = 0.04).
Figure 6
Blocking the neuronal, but not the glial, glutamate transporter can restore evoked network activity in the presence of the D1/D5 stimulation. (A) The superimposed traces on the left show that the glial glutamate transporter inhibitor trans-PDC (6 μM; 10 minutes) cannot restore evoked network activity in the presence of SKF 38393 (100 nM). The traces on the right show that TBOA (6 μM; 5 minutes), which also blocks the neuronal glutamate transporter, can restore evoked network activity in the presence of a maximally-suppressing level of SKF 38393. (B) Bar chart shows the mean ± S.E. for the area of the fast EPSC and the excitatory component of evoked network activity (late EPSCs) during application of SKF 38393 (100 nM) in the presence of trans-PDC (6-30 μM; 10 minutes; N = 6) or TBOA (3-6 μM; 3-10 minutes; N = 5). * denotes P < 0.01.
Similar articles
- Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro.
Gorelova NA, Yang CR. Gorelova NA, et al. J Neurophysiol. 2000 Jul;84(1):75-87. doi: 10.1152/jn.2000.84.1.75. J Neurophysiol. 2000. PMID: 10899185 - Activation of spinal d1/d5 receptors induces late-phase LTP of C-fiber-evoked field potentials in rat spinal dorsal horn.
Yang HW, Zhou LJ, Hu NW, Xin WJ, Liu XG. Yang HW, et al. J Neurophysiol. 2005 Aug;94(2):961-7. doi: 10.1152/jn.01324.2004. Epub 2005 Apr 13. J Neurophysiol. 2005. PMID: 15829590 - Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors.
Lambe EK, Aghajanian GK. Lambe EK, et al. Neuropsychopharmacology. 2006 Aug;31(8):1682-9. doi: 10.1038/sj.npp.1300944. Epub 2005 Nov 2. Neuropsychopharmacology. 2006. PMID: 16292328 - D5 dopamine receptor knockout mice and hypertension.
Yang Z, Sibley DR, Jose PA. Yang Z, et al. J Recept Signal Transduct Res. 2004 Aug;24(3):149-64. doi: 10.1081/rrs-200029971. J Recept Signal Transduct Res. 2004. PMID: 15521360 Review.
Cited by
- Molecular Pathways of the Therapeutic Effects of Ayahuasca, a Botanical Psychedelic and Potential Rapid-Acting Antidepressant.
Rossi GN, Guerra LTL, Baker GB, Dursun SM, Saiz JCB, Hallak JEC, Dos Santos RG. Rossi GN, et al. Biomolecules. 2022 Nov 2;12(11):1618. doi: 10.3390/biom12111618. Biomolecules. 2022. PMID: 36358968 Free PMC article. Review. - International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function.
Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S, Claeysen S, Cunningham KA, Fone KC, Gershon M, Di Giovanni G, Goodfellow NM, Halberstadt AL, Hartley RM, Hassaine G, Herrick-Davis K, Hovius R, Lacivita E, Lambe EK, Leopoldo M, Levy FO, Lummis SCR, Marin P, Maroteaux L, McCreary AC, Nelson DL, Neumaier JF, Newman-Tancredi A, Nury H, Roberts A, Roth BL, Roumier A, Sanger GJ, Teitler M, Sharp T, Villalón CM, Vogel H, Watts SW, Hoyer D. Barnes NM, et al. Pharmacol Rev. 2021 Jan;73(1):310-520. doi: 10.1124/pr.118.015552. Pharmacol Rev. 2021. PMID: 33370241 Free PMC article. Review. - Serotonin-2C and -2a receptor co-expression on cells in the rat medial prefrontal cortex.
Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA. Nocjar C, et al. Neuroscience. 2015 Jun 25;297:22-37. doi: 10.1016/j.neuroscience.2015.03.050. Epub 2015 Mar 27. Neuroscience. 2015. PMID: 25818050 Free PMC article. - Recent advances in the neuropsychopharmacology of serotonergic hallucinogens.
Halberstadt AL. Halberstadt AL. Behav Brain Res. 2015 Jan 15;277:99-120. doi: 10.1016/j.bbr.2014.07.016. Epub 2014 Jul 15. Behav Brain Res. 2015. PMID: 25036425 Free PMC article. Review.
References
- Abekawa T, Ohmori T, Ito K, Koyama T. D1 dopamine receptor activation reduces extracellular glutamate and GABA concentrations in the medial prefrontal cortex. Brain Res. 2000;867:250–4. - PubMed
- Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20:15–27. - PubMed
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. - PubMed
- Alburges ME, Hunt ME, McQuade RD, Wamsley JK. D1-receptor antagonists: comparison of [3H]SCH39166 to [3H]SCH23390. J Chem Neuroanat. 1992;5:357–66. - PubMed
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources