Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission - PubMed (original) (raw)
Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission
Nathan M Sherer et al. Nat Cell Biol. 2007 Mar.
Abstract
The spread of retroviruses between cells is estimated to be 2-3 orders of magnitude more efficient when cells can physically interact with each other. The underlying mechanism is largely unknown, but transfer is believed to occur through large-surface interfaces, called virological or infectious synapses. Here, we report the direct visualization of cell-to-cell transmission of retroviruses in living cells. Our results reveal a mechanism of virus transport from infected to non-infected cells, involving thin filopodial bridges. These filopodia originate from non-infected cells and interact, through their tips, with infected cells. A strong association of the viral envelope glycoprotein (Env) in an infected cell with the receptor molecules in a target cell generates a stable bridge. Viruses then move along the outer surface of the filopodial bridge toward the target cell. Our data suggest that retroviruses spread by exploiting an inherent ability of filopodia to transport ligands from cell to cell.
Figures
Figure 1
MLV moves from cell-to-cell along filopodial bridges. (A) Cos-1 cells generating infectious MLV labelled with Gag–CFP (green) and Env–YFP (red) were cocultured with target XC cells expressing mCAT1–CFP (green) and monitored by time-lapse microscopy. To illustrate the overall movement of viruses from cell to cell, 22 frames of the Supplementary Information, Movie 1 were superimposed. Arrows indicate the paths of five viral particles (a–e) undergoing transmission. (B) Single-particle tracking of particles a–e (shown in A) moving from the infected cell towards the non-infected target cells. (C) Average rates of particle movement, average transmission time and average distance travelled for 117 MLV particles undergoing cytonemal transmission. (D) Two arrays of individual frames from a time-lapse video covering approximately 15 min compare the stability of a filopodial bridge (upper panel; red, mCAT1–YFP) transporting viruses (green, MLV Gag–CFP) to that of an unanchored filopodium (lower panel). (E) Graphs indicating the lengths of five filopodial bridges compared with unanchored filopodia over time. The dotted lines represent the average length of either structure (5.8 ± 2.84 μm, n = 59 versus 2.37 ± 1.67 μm, n = 60). The scale bars represent 10 μm in A and 5 μm in D.
Figure 2
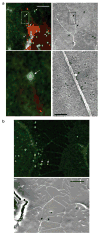
Viruses move along the outer surface of filopodial bridges toward target cells. (a, b) Fluorescently labelled viral particles (green, MLV Gag–CFP; white arrows) moving along filopodial bridges (red, mCAT1–YFP) correlated to single approximately 100-nm particles observed by scanning electron microscopy (black arrows). The boxed areas in the upper panels in a are magnified in the lower panels. The scale bars represent 5 μm in the upper panel in a and in b, and 500 nm in the lower panel in a.
Figure 3
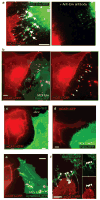
Viral cytoneme formation depends on Env–receptor interactions. (a) An MLV infected Cos-1 cell expressing MLV Env–YFP (green) in coculture with an XC cell expressing mCAT1–YFP (red). The arrows indicate receptor–Env accumulation at filopodial contacts. The right panel shows the same area after 1 h treatment with neutralizing antibodies directed against the extracellular domain of MLV Env. (b) An MLV infected Cos-1 cell expressing unlabelled wild-type Env in coculture with an XC cell expressing mCAT1–CFP (red). To visualize the plasma membrane of virus producing cells, palmitylated YFP (palm–YFP, green) was coexpressed. Cytonemal contacts are indicated by white arrows. (c–e) Experiments as in b showing the behaviour of cell-cell contacts in the absence of Env expression (c), the expression of a mutant Env impaired in receptor-binding (S84I, d) or the expression of a mutant Env (ΔH8, e) capable of receptor-binding, but impaired in a post-binding step. In e, infected cells were identified by coexpression of the endocytic marker dynamin 2–YFP. (f) HEK 293 cells expressing MLV Env–CFP (green) in coculture with an XC cell expressing receptor mCAT1–YFP (red). Arrows indicated sites of Env–receptor accumulation. Panels on the right show unmerged images. The scale bars represent 5 μm in a–f.
Figure 4
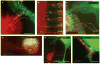
Viral cytoneme formation is driven by endocytic forces in the infected cell. (a) An XC cell expressing fluorescent receptor mCAT1–YFP (red) in contact with an XC-cell generating MLV labelled with Gag–CFP (green). The images are frames (time, min:s) from the Supplementary Information, Movie 3. The arrow indicates a lengthening filopodium. (b) An XC cell expressing receptor mCAT1–YFP (lower cell, red) in coculture with a Cos-1 cell generating fluorescently labelled MLV virions (upper cell; MLV Gag–CFP, green). The asterisks indicate membrane fragments containing mCAT1–YFP (red) recently released from the tips of filopodia. The entire time-lapse can be viewed in the Supplementary Information, Movie 4. (c–e) XC cells expressing variants of mCAT1–GFP fusion proteins (red) in coculture with MLV–infected Cos-1 cells expressing either dynamin 2–CFP or caveolin1–YFP (green), as indicated. Cells in e were incubated with Alexa Fluor 555-labelled cholera toxin B subunit for 10 min to label GM1 (blue). Arrows indicate the accumulation of these proteins at sites of cytonemal contact. The boxed areas in c and d are shown as unmerged images on the right of these panels. (f) Transmission electron micrograph of a cell–cell contact observed in a coculture of infected Cos-1 cells (right) with receptor-expressing XC cells (left). The scale bars represent 5 μm in a–e and 200 nm in f.
Figure 5
Cytonemes mediate cell-to-cell transmission of retroviruses. (a) Frames from time-lapse imaging, as described in Fig. 1a, were superimposed. The two arrows illustrate the opposing movements at cytonemal contacts — the lengthening and internalization of receptor-expressing filopodium (red, mCAT1–YFP) and the movement of viral particles (green, MLV Gag–CFP). The entire sequence is shown in the Supplementary Information, Movie 6. (b) Enlargements of individual frames of the experiment shown in Fig. 1d to illustrate the concentration of receptor (red, mCAT1–YFP) beneath the moving viral particle (green, MLV Gag–CFP). (c) Coculture of infected Cos-1 cells generating MLV labelled with Gag–YFP (red) with XC cells that express (green, mCAT1–CFP) or do not express additional viral receptor (white lines in cell expressing unlabelled endogenous mCAT1). The image represents 69 superimposed frames from a 69 min time-lapse movie. Paths of virus transfer are indicated by arrows. (d) An XC cell chronically infected with MLV efficiently transmits virus particles to target cells in the absence of additional viral Env expression. The region of interest is also shown in the Supplementary Information, Movie 2. (e, f) Cytoneme formation and particle transport were also observed for the envelope–receptor combinations HIV-1 Env–CD4–YFP–CXCR4 (see Supplementary Information, Movie 7) and ALV EnvA–receptor Tva–YFP as indicated. The scale bars represent 5 μm in a and c and 10 μm in e and f.
Comment in
- Bridging efficient viral infection.
Hope TJ. Hope TJ. Nat Cell Biol. 2007 Mar;9(3):243-4. doi: 10.1038/ncb0307-243. Nat Cell Biol. 2007. PMID: 17330113 No abstract available.
Similar articles
- Bridging efficient viral infection.
Hope TJ. Hope TJ. Nat Cell Biol. 2007 Mar;9(3):243-4. doi: 10.1038/ncb0307-243. Nat Cell Biol. 2007. PMID: 17330113 No abstract available. - Green fluorescent protein-tagged retroviral envelope protein for analysis of virus-cell interactions.
Spitzer D, Dittmar KE, Rohde M, Hauser H, Wirth D. Spitzer D, et al. J Virol. 2003 May;77(10):6070-5. doi: 10.1128/jvi.77.10.6070-6075.2003. J Virol. 2003. PMID: 12719600 Free PMC article. - Interactions of CCR5 and CXCR4 with CD4 and gp120 in human blood monocyte-derived dendritic cells.
Xiao X, Kinter A, Broder CC, Dimitrov DS. Xiao X, et al. Exp Mol Pathol. 2000 Jun;68(3):133-8. doi: 10.1006/exmp.1999.2300. Exp Mol Pathol. 2000. PMID: 10816381 - Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins.
Overbaugh J, Miller AD, Eiden MV. Overbaugh J, et al. Microbiol Mol Biol Rev. 2001 Sep;65(3):371-89, table of contents. doi: 10.1128/MMBR.65.3.371-389.2001. Microbiol Mol Biol Rev. 2001. PMID: 11528001 Free PMC article. Review. - Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis.
Sherer NM, Mothes W. Sherer NM, et al. Trends Cell Biol. 2008 Sep;18(9):414-20. doi: 10.1016/j.tcb.2008.07.003. Epub 2008 Aug 14. Trends Cell Biol. 2008. PMID: 18703335 Free PMC article. Review.
Cited by
- 6-Shogaol Abrogates Parkinson's Disease in Rotenone-Induced Rodents: Based on In Silico Study and Inhibiting TNF-α/NF-κB/IL-1β/MAO-B.
Rafeeq M, Al-Abbasi FA, Afzal M, Moglad E, Al-Qahtani SD, Alzrea SI, Almalki NAR, Imam F, Sayyed N, Kazmi I. Rafeeq M, et al. Pharmaceuticals (Basel). 2024 Oct 9;17(10):1348. doi: 10.3390/ph17101348. Pharmaceuticals (Basel). 2024. PMID: 39458989 Free PMC article. - The KT Jeang retrovirology prize 2024: Walther Mothes.
Mothes W. Mothes W. Retrovirology. 2024 Oct 24;21(1):16. doi: 10.1186/s12977-024-00649-8. Retrovirology. 2024. PMID: 39449025 Free PMC article. No abstract available. - Utilizing Adenovirus Knob Proteins as Carriers in Cancer Gene Therapy Amidst the Presence of Anti-Knob Antibodies.
Koizumi N, Hirai T, Kano J, Sato A, Suzuki Y, Sasaki A, Nomura T, Utoguchi N. Koizumi N, et al. Int J Mol Sci. 2024 Oct 3;25(19):10679. doi: 10.3390/ijms251910679. Int J Mol Sci. 2024. PMID: 39409008 Free PMC article. - Intercellular Transport of Viral Proteins.
Simon F, Thoma-Kress AK. Simon F, et al. Results Probl Cell Differ. 2024;73:435-474. doi: 10.1007/978-3-031-62036-2_18. Results Probl Cell Differ. 2024. PMID: 39242389 Review. - Tunneling Nanotubes: The Cables for Viral Spread and Beyond.
Kapoor D, Sharma P, Saini A, Azhar E, Elste J, Kohlmeir EK, Shukla D, Tiwari V. Kapoor D, et al. Results Probl Cell Differ. 2024;73:375-417. doi: 10.1007/978-3-031-62036-2_16. Results Probl Cell Differ. 2024. PMID: 39242387 Review.
References
- Carr JM, Hocking H, Li P, Burrell CJ. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology. 1999;265:319–329. - PubMed
- Igakura T, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. - PubMed
- McDonald D, et al. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources