The power to reduce: pyridine nucleotides--small molecules with a multitude of functions - PubMed (original) (raw)
Review
The power to reduce: pyridine nucleotides--small molecules with a multitude of functions
Nadine Pollak et al. Biochem J. 2007.
Abstract
The pyridine nucleotides NAD and NADP play vital roles in metabolic conversions as signal transducers and in cellular defence systems. Both coenzymes participate as electron carriers in energy transduction and biosynthetic processes. Their oxidized forms, NAD+ and NADP+, have been identified as important elements of regulatory pathways. In particular, NAD+ serves as a substrate for ADP-ribosylation reactions and for the Sir2 family of NAD+-dependent protein deacetylases as well as a precursor of the calcium mobilizing molecule cADPr (cyclic ADP-ribose). The conversions of NADP+ into the 2'-phosphorylated form of cADPr or to its nicotinic acid derivative, NAADP, also result in the formation of potent intracellular calcium-signalling agents. Perhaps, the most critical function of NADP is in the maintenance of a pool of reducing equivalents which is essential to counteract oxidative damage and for other detoxifying reactions. It is well known that the NADPH/NADP+ ratio is usually kept high, in favour of the reduced form. Research within the past few years has revealed important insights into how the NADPH pool is generated and maintained in different subcellular compartments. Moreover, tremendous progress in the molecular characterization of NAD kinases has established these enzymes as vital factors for cell survival. In the present review, we summarize recent advances in the understanding of the biosynthesis and signalling functions of NAD(P) and highlight the new insights into the molecular mechanisms of NADPH generation and their roles in cell physiology.
Figures
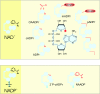
Figure 1. Schematic overview of NAD(P) biosynthetic pathways
The major known pathways of NAD(P) synthesis are presented. Main endogenous precursors of NAD(P) synthesis are Nam, NA and
L
-tryptophan. Nicotinamidase is only found in yeast. NamR, Nam riboside; NA/NamPRT, NA/Nam phosphoribosyltransferase; NADS, NAD synthetase.
Figure 2. Signalling derivatives of NAD(P)
The ADP-ribosyl moiety commonly shared by all derivatives is illustrated in structural detail and adumbrated in light blue, the red asterisk indicates the site of ADP-ribosyl attachment to the acceptor. The individual portion of each derivative is presented in red and the 2′-phosphate group of NADP+ in its derivatives is shown in dark blue. ADPr, ADP-ribose; mAPDr, mono-ADPr; pADPr, poly-ADPr; 2′P-cADPr, 2′-phosphate cADPr.
Figure 3. Generation and utilization of NADPH in eukaryotic cells
Cyt P450, cytochrome P450; Trx, thioredoxin.
Figure 4. Partial multiple sequence alignment of NADKs from several organisms
Amino acid sequences of human NADK (protein ID NP_075394); A. thaliana NADK-1, NADK-2 and NADK-3 (NP_974347, NP_564145 and NP_177980); S. cerevisiae NADK-1/Utr1p, NADK-2/Yef1p and NADK-3/Pos5p (P21373, NP_010873 and NP_015136); E. coli YfjB (NP_417105); and M. tuberculosis Ppnk (BAB21478) were compared using Clustal W [170]. Identical and similar residues are highlighted in black and grey respectively. The two conserved NADK motifs are boxed in red.
Figure 5. Subcellular distribution of NADP metabolism in eukaryotic cells
NADK isoforms described in mammals, yeast and Arabidopsis are highlighted in blue, red and green respectively. Transhydrogenase and ADP-ribosyl cyclase have not been detected in yeast.
Similar articles
- New functions of a long-known molecule. Emerging roles of NAD in cellular signaling.
Ziegler M. Ziegler M. Eur J Biochem. 2000 Mar;267(6):1550-64. doi: 10.1046/j.1432-1327.2000.01187.x. Eur J Biochem. 2000. PMID: 10712584 Review. - The new life of a centenarian: signalling functions of NAD(P).
Berger F, Ramírez-Hernández MH, Ziegler M. Berger F, et al. Trends Biochem Sci. 2004 Mar;29(3):111-8. doi: 10.1016/j.tibs.2004.01.007. Trends Biochem Sci. 2004. PMID: 15003268 Review. - The phosphate makes a difference: cellular functions of NADP.
Agledal L, Niere M, Ziegler M. Agledal L, et al. Redox Rep. 2010;15(1):2-10. doi: 10.1179/174329210X12650506623122. Redox Rep. 2010. PMID: 20196923 Free PMC article. Review. - NAD - new roles in signalling and gene regulation in plants.
Hunt L, Lerner F, Ziegler M. Hunt L, et al. New Phytol. 2004 Jul;163(1):31-44. doi: 10.1111/j.1469-8137.2004.01087.x. New Phytol. 2004. PMID: 33873776 Review. - Intramolecular ADP-ribose transfer reactions and calcium signalling. Potential role of 2'-phospho-cyclic ADP-ribose in oxidative stress.
Vu CQ, Coyle DL, Tai HH, Jacobson EL, Jacobson MK. Vu CQ, et al. Adv Exp Med Biol. 1997;419:381-8. Adv Exp Med Biol. 1997. PMID: 9193680 Review.
Cited by
- Expression and Functional Analysis of the Propamocarb-Related Gene CsMCF in Cucumber.
Zhang F, Xin M, Yu S, Liu D, Zhou X, Qin Z. Zhang F, et al. Front Plant Sci. 2019 Jul 4;10:871. doi: 10.3389/fpls.2019.00871. eCollection 2019. Front Plant Sci. 2019. PMID: 31333707 Free PMC article. - Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix.
Niere M, Kernstock S, Koch-Nolte F, Ziegler M. Niere M, et al. Mol Cell Biol. 2008 Jan;28(2):814-24. doi: 10.1128/MCB.01766-07. Epub 2007 Nov 8. Mol Cell Biol. 2008. PMID: 17991898 Free PMC article. - States of quinolinic acid excess in urine: A systematic review of human studies.
Saade MC, Clark AJ, Parikh SM. Saade MC, et al. Front Nutr. 2022 Dec 16;9:1070435. doi: 10.3389/fnut.2022.1070435. eCollection 2022. Front Nutr. 2022. PMID: 36590198 Free PMC article. - G6PD-mediated increase in de novo NADP+ biosynthesis promotes antioxidant defense and tumor metastasis.
Zhang Y, Xu Y, Lu W, Li J, Yu S, Brown EJ, Stanger BZ, Rabinowitz JD, Yang X. Zhang Y, et al. Sci Adv. 2022 Jul 22;8(29):eabo0404. doi: 10.1126/sciadv.abo0404. Epub 2022 Jul 20. Sci Adv. 2022. PMID: 35857842 Free PMC article. - Biochemical Alterations in Triticale Seedlings Pretreated with Selective Herbicide and Subjected to Drought or Waterlogging Stress.
Katerova Z, Todorova D, Shopova E, Brankova L, Dimitrova L, Petrakova M, Sergiev I. Katerova Z, et al. Plants (Basel). 2023 Jul 28;12(15):2803. doi: 10.3390/plants12152803. Plants (Basel). 2023. PMID: 37570956 Free PMC article.
References
- Berger F., Ramirez-Hernandez M. H., Ziegler M. The new life of a centenarian: signalling functions of NAD(P) Trends Biochem. Sci. 2004;29:111–118. - PubMed
- Magni G., Amici A., Emanuelli M., Raffaelli N., Ruggieri S. Enzymology of NAD+ synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1999;73:135–182. - PubMed
- Berger F., Lau C., Dahlmann M., Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 2005;280:36334–36341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous