Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting - PubMed (original) (raw)
Comparative Study
. 2007 Feb 27;104(9):3219-24.
doi: 10.1073/pnas.0611206104. Epub 2007 Feb 12.
Affiliations
- PMID: 17296940
- PMCID: PMC1805530
- DOI: 10.1073/pnas.0611206104
Comparative Study
Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting
I B Lobov et al. Proc Natl Acad Sci U S A. 2007.
Abstract
Genetic deletion studies have shown that haploinsufficiency of Delta-like ligand (Dll) 4, a transmembrane ligand for the Notch family of receptors, results in major vascular defects and embryonic lethality. To better define the role of Dll4 during vascular growth and differentiation, we selected the postnatal retina as a model because its vasculature develops shortly after birth in a highly stereotypic manner, during which time it is accessible to experimental manipulation. We report that Dll4 expression is dynamically regulated by VEGF in the retinal vasculature, where it is most prominently expressed at the leading front of actively growing vessels. Deletion of a single Dll4 allele or pharmacologic inhibition of Dll4/Notch signaling by intraocular administration of either soluble Dll4-Fc or a blocking antibody against Dll4 all produced the same set of characteristic abnormalities in the developing retinal vasculature, most notably enhanced angiogenic sprouting and increased endothelial cell proliferation, resulting in the formation of a denser and more highly interconnected superficial capillary plexus. In a model of ischemic retinopathy, Dll4 blockade also enhanced angiogenic sprouting and regrowth of lost retinal vessels while suppressing ectopic pathological neovascularization. Our data demonstrate that Dll4 is induced by VEGF as a negative feedback regulator and acts to prevent overexuberant angiogenic sprouting, promoting the timely formation of a well differentiated vascular network.
Conflict of interest statement
Conflict of interest statement: I.B.L., R.A.R., N.P., N.W.G., G.T., G.D.Y., and S.J.W. are employees of, and own stock or stock options in, Regeneron Pharmaceuticals.
Figures
Fig. 1.
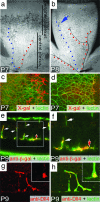
Dll4 expression in the developing retinal vasculature. (a and b) X-gal staining of the developing superficial retinal vasculature in Dll4+/lacZ mice at P7 (a) and P8 (b). Note the highest level of Dll4 expression in arteries (red dots) and lower levels of expression in the forming vein (blue dots) and capillaries in the area proximal to the edge of the superficial plexus (blue arrow). (c and d) Dll4 reporter gene (c, X-gal staining, red pseudocolor) and Dll4 protein (d, red) expression in the leading front of the superficial retinal plexus in Dll4+/lacZ (c) or wild-type (d) mice at P7. Green, GS lectin. (e and f) Dll4 reporter gene expression in tips (red filled arrow) and stalks (white arrows) of growing deep retinal vessels at P9. GS lectin (green) and anti-β-gal (red) staining are shown. (g and h) Dll4 immunostaining (red) in tips (red filled arrows) and stalks (white arrows) of deep retinal sprouts in wild-type mice at P9. (Inset) Higher power view of sprout tip. Green, GS lectin. (Original magnifications: ×40 for a and b, ×100 for c and d, ×400 for e, and ×800 for f–h.)
Fig. 2.
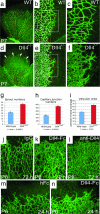
Retinal vascular abnormalities in Dll4+/lacZ mice. (a–f) GS lectin staining of the developing retinal vessels in wild-type (a–c) and Dll4+/lacZ (d–f) mice at P7. (a and d) Delayed capillary remodeling and formation of syncytium-like vascular plexus in retinas of Dll4+/lacZ mice. Red dots mark arteries, and yellow dots mark veins. White arrows indicate syncytium-like vascular plexus. (b and e) Increased sprouting and capillary network complexity in the superficial retinal vasculature of Dll4+/lacZ mice. Red dots mark tips of sprouts, and white dots mark intercapillary junctions. (c and f) Filopodia are substantially more numerous at the leading edge of the growing retinal vasculature in Dll4+/lacZ mice. (g–i) Quantitation of sprout (g) and capillary junction (h) numbers and vascular area (i) in ×100 microscopy fields. Error bars represent standard errors. (j–l) GS lectin staining of the developing retinal vasculature of wild-type mice 3 days after intravitreal injection of 0.5 μg of control IgG (j), Dll4-Fc (k), or anti-Dll4 antibody (l). Dll4-Fc or anti-Dll4 antibody administration to wild-type mice produced vascular abnormalities similar to those seen in Dll4+/lacZ mice. Note the very dense capillary network. (m and n) Intravitreal injection of 0.8 μg of Dll4-Fc (m) in wild-type mice at P5 induces formation of the syncytium-like vascular plexus within 24 h. (Original magnifications: ×40 for a and d, ×100 for j–n, and ×200 for c and f.)
Fig. 3.
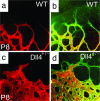
Perfusion staining of developing retinal vasculature in Dll4 mice. (a–d) Perfusion staining with L. esculentum lectin (a and c) and counterstaining with GS lectin (green, b and d) of the developing retinal vasculature in wild-type (a and b) and Dll4+/lacZ (c and d) mice at P8. (Original magnification: ×630.)
Fig. 4.
Effect of Dll4 pharmacological inhibition on the developing retinal vasculature and proliferation. (a and b) BrdU labeling of retinal vasculature in hFc-treated (a) and Dll4-Fc-treated (b) retinas. Counterstaining with anti-VE-Cadherin antibody is shown. (c) Quantification of BrdU-positive nuclei numbers per half of ×20 microscopy fields. (d and e) Real-time PCR analysis of VEGF-A (d) and VEGFR2 (e) gene expression in the retinas treated for 24 h with hFc or Dll4-Fc. Expression data were normalized to GAPDH. Error bars represent standard errors. (f and g) VEGFR2 antibody staining of the leading front of the growing retinal vasculature treated for 24 h with hFc (f) or Dll4-Fc (g). (Original magnifications: ×200 for a and b and ×400 for f and g.)
Fig. 5.
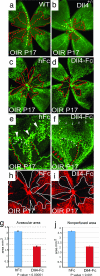
Effect of Dll4 inhibition on normal retinal vascular development and neovascularization in OIR model. (a and b) GS lectin staining of OIR retinas at P17 from wild-type (a) and Dll4+/lacZ (b) mice. Note smaller avascular area (red line) and increased vessel density in Dll4+/lacZ retina. (c and d) GS lectin staining of OIR retinas at P17 from wild-type mice injected at P13 with 0.5 μg of hFc (c) or Dll4-Fc (d). Note the smaller avascular zone (red line) in the Dll4-Fc-treated retinas. (e and f) GS lectin staining of OIR retinas at P17 from wild-type mice injected at P13 with 0.5 μg of hFc (e) or Dll4-Fc (f). Note that most of sprouting (red arrows) occurs from veins (yellow dots) and capillaries. Also, note the reduced number of neovascular tufts (white arrowheads) and substantially denser capillary network (small white arrows) in Dll4-Fc-treated retinas. White dots indicate arteriovenous shunts whose appearance is reduced in Dll4-Fc-treated retinas. (g and j) Quantification of avascular (g) and nonperfused (j) area in hFc- and Dll4-Fc-treated P17 OIR retinas. Error bars represent standard errors. (h and i) Perfusion staining with L. esculentum lectin of the developing retinal vasculature in P17 OIR mice injected at P13 with 0.5 μg of hFc (h) or Dll4-Fc (i) at P17. (Original magnifications: ×20 for a–d and ×40 for e, f, h, and i.)
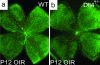
Fig. 6.
Reduced oxygen-induced vasoobliteration in the central retina in Dll4+/lacZ mice. Shown is GS lectin staining of the retinal vasculature in wild-type (a) and Dll4+/lacZ (b) OIR mice at P12. Note that most of the capillary pruning in wild-type retinas occurs in the vicinity of arteries (labeled with red dots). (Original magnification: ×20.)
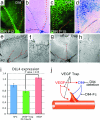
Fig. 7.
VEGF induces Dll4 expression in angiogenic sprouts. (a–d) GS lectin (a and c) and X-gal staining (b and d) of P12 (a and b) and P15 (c and d) OIR retinas from Dll4+/lacZ mice. Note strong Dll4 gene reporter expression in the vein (yellow and blue dots) and angiogenic sprouts in OIR retinas at P15. (e–h) X-gal staining of retinal vasculature from P7 Dll4+/lacZ mice treated for 48 h with 300 ng of hFc (e and f) or VEGF Trap (g and h). Blocking VEGF markedly reduces Dll4 expression in the tip and stalk endothelial cells. (i) Real-time PCR analysis of Dll4 expression in the retinas treated for 24 h with hFc, VEGF Trap, or VEGF165. Dll4 expression data were normalized to GAPDH. Error bars represent standard errors. (j) Interaction between VEGF and Dll4 in angiogenic sprouting. VEGF initiates proliferation and blood vessel sprouting and guides growing vessels. At the same time VEGF induces expression of Dll4 that blocks excessive proliferation and sprouting. (Original magnifications: ×40 for e and g, ×100 for a–d, and ×200 for f and h.)
Similar articles
- The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching.
Suchting S, Freitas C, le Noble F, Benedito R, Bréant C, Duarte A, Eichmann A. Suchting S, et al. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3225-30. doi: 10.1073/pnas.0611177104. Epub 2007 Feb 12. Proc Natl Acad Sci U S A. 2007. PMID: 17296941 Free PMC article. - Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis.
Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Hellström M, et al. Nature. 2007 Feb 15;445(7129):776-80. doi: 10.1038/nature05571. Epub 2007 Jan 28. Nature. 2007. PMID: 17259973 - Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis.
Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Noguera-Troise I, et al. Nature. 2006 Dec 21;444(7122):1032-7. doi: 10.1038/nature05355. Nature. 2006. PMID: 17183313 - Delta-like 4/Notch signaling and its therapeutic implications.
Yan M, Plowman GD. Yan M, et al. Clin Cancer Res. 2007 Dec 15;13(24):7243-6. doi: 10.1158/1078-0432.CCR-07-1393. Clin Cancer Res. 2007. PMID: 18094402 Review. - VEGFRs and Notch: a dynamic collaboration in vascular patterning.
Jakobsson L, Bentley K, Gerhardt H. Jakobsson L, et al. Biochem Soc Trans. 2009 Dec;37(Pt 6):1233-6. doi: 10.1042/BST0371233. Biochem Soc Trans. 2009. PMID: 19909253 Review.
Cited by
- TRIM28 regulates sprouting angiogenesis through VEGFR-DLL4-Notch signaling circuit.
Wang Y, Singh AR, Zhao Y, Du T, Huang Y, Wan X, Mukhopadhyay D, Wang Y, Wang N, Zhang P. Wang Y, et al. FASEB J. 2020 Nov;34(11):14710-14724. doi: 10.1096/fj.202000186RRR. Epub 2020 Sep 12. FASEB J. 2020. PMID: 32918765 Free PMC article. - Hedgehog regulates angiogenesis of intersegmental vessels through the VEGF signaling pathway.
Moran CM, Myers CT, Lewis CM, Krieg PA. Moran CM, et al. Dev Dyn. 2012 Jun;241(6):1034-42. doi: 10.1002/dvdy.23795. Epub 2012 May 2. Dev Dyn. 2012. PMID: 22513894 Free PMC article. - Endothelial epsins as regulators and potential therapeutic targets of tumor angiogenesis.
Song K, Wu H, Rahman HN, Dong Y, Wen A, Brophy ML, Wong S, Kwak S, Bielenberg DR, Chen H. Song K, et al. Cell Mol Life Sci. 2017 Feb;74(3):393-398. doi: 10.1007/s00018-016-2347-2. Epub 2016 Aug 29. Cell Mol Life Sci. 2017. PMID: 27572288 Free PMC article. Review. - Inhibition of tumor angiogenesis and tumor growth by the DSL domain of human Delta-like 1 targeted to vascular endothelial cells.
Zhao XC, Dou GR, Wang L, Liang L, Tian DM, Cao XL, Qin HY, Wang CM, Zhang P, Han H. Zhao XC, et al. Neoplasia. 2013 Jul;15(7):815-25. doi: 10.1593/neo.13550. Neoplasia. 2013. PMID: 23814493 Free PMC article. - Novel insights into the differential functions of Notch ligands in vascular formation.
Kume T. Kume T. J Angiogenes Res. 2009 Nov 16;1:8. doi: 10.1186/2040-2384-1-8. J Angiogenes Res. 2009. PMID: 20016694 Free PMC article.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Science. 1999;284:770–776. - PubMed
- Shawber CJ, Kitajewski J. BioEssays. 2004;26:225–234. - PubMed
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Hum Mol Genet. 1999;8:723–730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous