Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla - PubMed (original) (raw)
Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla
A Kangarlu et al. AJNR Am J Neuroradiol. 2007 Feb.
Abstract
Background and purpose: Conventional imaging of ex-vivo brain at 1.5T in multiple sclerosis (MS) detects only a small fraction of the gray matter cerebral cortical lesions that can be detected by pathology. Our purpose was to examine if imaging at 8T can detect plaques in cortical gray matter (CGM) not evident at 1.5T.
Methods: An ex-vivo brain obtained at autopsy from a patient with MS was formalin fixed and 1 cm coronal slices were examined using MR imaging at 8T.
Results: Numerous cerebral cortical lesions not evident at 1.5T were seen at 8T. Lesions were easily identified using gradient-echo and spin-echo (SE) as well as diffusion images. MR imaging at 8T identified many of the types of plaques previously evident only by pathology. The magnitude of the cortical involvement in this 1 patient was severe. Lesions in the gray matter readily visible by high-field MR imaging were sometimes barely visible by pathology. MR imaging at 8T often facilitated the detection of such plaques by pathology.
Conclusion: This study establishes the utility of high-field imaging at 8T in the delineation of plaques in the cerebral CGM in MS.
Figures
Fig 1.
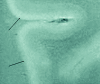
A, SE T2-weighted image, TR = 750 ms, TE = 20 ms, FOV = 15 cm, matrix = 1024 × 1024, and section thickness = 1 mm. B, Luxol fast blue stain at approximately 400×. Type 1 cortical lesion. The lesion involves the deeper cortical layers and is clearly seen on MR imaging (A) and barely noticeable by pathology (B).
Fig 2.
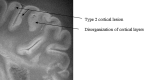
SE diffusion T2-weighted image, TR = 1000 ms, TE = 65 ms, FOV = 15 cm, matrix = 512 × 512, and thickness = 1 mm. Type 2 cortical lesions. These cortical lesions involve all layers of the cortex, and the normal trilaminar appearance is lost. The subcortical white matter is unaffected. Disorganization of the cortical layers is the hallmark of this pattern.
Fig 3.
SE T2-weighted image, TR = 750 ms, TE = 20 ms, FOV = 15 cm, matrix = 1024 × 1024, and section thickness = 1 mm. Type 3 cortical lesions. Bandlike areas of demyelination along the outer cortical layers spanning adjacent gyri result in extensive involvement of the cortex.
Fig 4.
A, Luxol fast blue stain at approximately 400×. B, GRE T2-weighted image, TR = 500, TE = 11, FOV = 15 cm, matrix = 1024 × 1024, and section thickness = 1 mm. Type 4 cortical lesion. Involvement predominantly affects the subcortical U-fibers, resulting in a juxtacortical lesion extending into the cortex.
Fig 5.
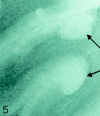
SE T2-weighted image, TR = 750 ms, TE = 20 ms, FOV = 15 cm, matrix = 1024 × 1024, section thickness = 1 mm. Type 5 cortical lesions (arrows). Lesion extends from all layers of the cortex to the adjacent white matter. The adjacent white-matter involvement distinguishes these lesions from type 2 lesions, which are restricted to the layers of the cortex only.
Fig 6.
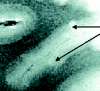
SE diffusion T2-weighted image, TR = 1000 ms, TE = 65 ms, FOV = 15 cm, matrix = 512 × 512, and section thickness = 1 mm. Type 6 cortical lesions. Small and multiple lesions that occur across the cortical ribbon in any layers of the cortex.
Fig 7.
A, GRE T2-weighted image, TR = 500, TE = 11, FOV = 15 cm, matrix = 1024 × 1024, and section thickness = 1 mm. Multiple patterns are evident within a region of interest in which 5 different patters of involvement of the cerebral cortex can be identified. B, Corresponding FLAIR T2-weighted image at 1.5T for comparison fails to demonstrate the cortical lesions noted in A at 8T.
Similar articles
- Does high-field MR imaging improve cortical lesion detection in multiple sclerosis?
Geurts JJ, Blezer EL, Vrenken H, van der Toorn A, Castelijns JA, Polman CH, Pouwels PJ, Bö L, Barkhof F. Geurts JJ, et al. J Neurol. 2008 Feb;255(2):183-91. doi: 10.1007/s00415-008-0620-5. Epub 2008 Feb 4. J Neurol. 2008. PMID: 18231704 - In vivo detection of cortical plaques by MR imaging in patients with multiple sclerosis.
Bagnato F, Butman JA, Gupta S, Calabrese M, Pezawas L, Ohayon JM, Tovar-Moll F, Riva M, Cao MM, Talagala SL, McFarland HF. Bagnato F, et al. AJNR Am J Neuroradiol. 2006 Nov-Dec;27(10):2161-7. AJNR Am J Neuroradiol. 2006. PMID: 17110688 Free PMC article. - Imaging gray matter with concomitant null point imaging from the phase sensitive inversion recovery sequence.
Mougin O, Abdel-Fahim R, Dineen R, Pitiot A, Evangelou N, Gowland P. Mougin O, et al. Magn Reson Med. 2016 Nov;76(5):1512-1516. doi: 10.1002/mrm.26061. Epub 2015 Nov 24. Magn Reson Med. 2016. PMID: 26599705 Free PMC article. - Cortical lesions in multiple sclerosis.
Calabrese M, Filippi M, Gallo P. Calabrese M, et al. Nat Rev Neurol. 2010 Aug;6(8):438-44. doi: 10.1038/nrneurol.2010.93. Epub 2010 Jul 13. Nat Rev Neurol. 2010. PMID: 20625376 Review. - Gray and normal-appearing white matter in multiple sclerosis: an MRI perspective.
Vrenken H, Geurts JJ. Vrenken H, et al. Expert Rev Neurother. 2007 Mar;7(3):271-9. doi: 10.1586/14737175.7.3.271. Expert Rev Neurother. 2007. PMID: 17341175 Review.
Cited by
- Clinical correlates of grey matter pathology in multiple sclerosis.
Horakova D, Kalincik T, Dusankova JB, Dolezal O. Horakova D, et al. BMC Neurol. 2012 Mar 7;12:10. doi: 10.1186/1471-2377-12-10. BMC Neurol. 2012. PMID: 22397707 Free PMC article. Review. - MRI in multiple sclerosis: what's inside the toolbox?
Neema M, Stankiewicz J, Arora A, Guss ZD, Bakshi R. Neema M, et al. Neurotherapeutics. 2007 Oct;4(4):602-17. doi: 10.1016/j.nurt.2007.08.001. Neurotherapeutics. 2007. PMID: 17920541 Free PMC article. Review. - Medial temporal cortices in ex vivo magnetic resonance imaging.
Augustinack JC, van der Kouwe AJ, Fischl B. Augustinack JC, et al. J Comp Neurol. 2013 Dec 15;521(18):4177-88. doi: 10.1002/cne.23432. J Comp Neurol. 2013. PMID: 23881818 Free PMC article. Review. - Gray matter pathology and multiple sclerosis.
Wegner C, Stadelmann C. Wegner C, et al. Curr Neurol Neurosci Rep. 2009 Sep;9(5):399-404. doi: 10.1007/s11910-009-0058-x. Curr Neurol Neurosci Rep. 2009. PMID: 19664370 Review. - Oligodendroglia in cortical multiple sclerosis lesions decrease with disease progression, but regenerate after repeated experimental demyelination.
Rodriguez EG, Wegner C, Kreutzfeldt M, Neid K, Thal DR, Jürgens T, Brück W, Stadelmann C, Merkler D. Rodriguez EG, et al. Acta Neuropathol. 2014 Aug;128(2):231-46. doi: 10.1007/s00401-014-1260-8. Epub 2014 Feb 25. Acta Neuropathol. 2014. PMID: 24563023 Free PMC article.
References
- Lumsden CE. The Neuropathology of Multiple Sclerosis. Amsterdam: North-Holland;1970. :217–309
- Peterson JW, Bo L, Mork S, et al. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001;50:389–400 - PubMed
- Kidd D, Barkhof F, McConnell R, et al. Cortical lesions in multiple sclerosis. Brain 1999;122:17–26 - PubMed
- Sharma R, Narayana PA, Wolinsky JS. Grey matter abnormalities in multiple sclerosis. proton magnetic resonance spectroscopic imaging. Mult Scler 2001;7:221–26 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical