Epigenetic control of the foxp3 locus in regulatory T cells - PubMed (original) (raw)
doi: 10.1371/journal.pbio.0050038.
Jennifer Freyer, Christiane Siewert, Udo Baron, Sven Olek, Julia Polansky, Kerstin Schlawe, Hyun-Dong Chang, Tobias Bopp, Edgar Schmitt, Stefan Klein-Hessling, Edgar Serfling, Alf Hamann, Jochen Huehn
Affiliations
- PMID: 17298177
- PMCID: PMC1783672
- DOI: 10.1371/journal.pbio.0050038
Epigenetic control of the foxp3 locus in regulatory T cells
Stefan Floess et al. PLoS Biol. 2007 Feb.
Abstract
Compelling evidence suggests that the transcription factor Foxp3 acts as a master switch governing the development and function of CD4(+) regulatory T cells (Tregs). However, whether transcriptional control of Foxp3 expression itself contributes to the development of a stable Treg lineage has thus far not been investigated. We here identified an evolutionarily conserved region within the foxp3 locus upstream of exon-1 possessing transcriptional activity. Bisulphite sequencing and chromatin immunoprecipitation revealed complete demethylation of CpG motifs as well as histone modifications within the conserved region in ex vivo isolated Foxp3(+)CD25(+)CD4(+) Tregs, but not in naïve CD25(-)CD4(+) T cells. Partial DNA demethylation is already found within developing Foxp3(+) thymocytes; however, Tregs induced by TGF-beta in vitro display only incomplete demethylation despite high Foxp3 expression. In contrast to natural Tregs, these TGF-beta-induced Foxp3(+) Tregs lose both Foxp3 expression and suppressive activity upon restimulation in the absence of TGF-beta. Our data suggest that expression of Foxp3 must be stabilized by epigenetic modification to allow the development of a permanent suppressor cell lineage, a finding of significant importance for therapeutic applications involving induction or transfer of Tregs and for the understanding of long-term cell lineage decisions.
Conflict of interest statement
Competing interests. We herewith state a competing financial interest for the authors S. Olek and U. Baron, who are a founder and an employee of the company Epiontis, respectively.
Figures
Figure 1. Stable Foxp3 Expression in CD25+CD4+ Tregs
CD25+CD4+ Tregs were isolated ex vivo from pooled spleen and LN single-cell suspensions, labeled with CFSE, and then transferred into syngenic 8-wk-old recipients (2 × 106/mouse). Before transfer, sorted cells were analyzed for intracellular Foxp3 expression by FACS. Fourteen days after transfer, splenocytes of recipient mice were stained for CD4, CD25, and Foxp3, and analyzed by FACS. About 0.1% of total splenocytes and, accordingly, 3% of total Foxp3+ splenocytes were donor derived (CFSE+). Representative dot and histogram plots from four independently analyzed mice were selected. Numbers display frequency of cells within indicated populations. Comparable results were obtained for cells isolated from peripheral or mesenteric LNs (unpublished data). The bars in the left and right graphs indicate the marker gate for Foxp3+ cells. The box in the middle graph indicates the region that was used to gate for CD4+CFSE+ cells. These gated cells (CFSE+) are depicted in the right graph.
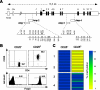
Figure 2. Selectively Demethylated CpG Motifs within the foxp3 Locus in CD25+CD4+ Tregs Isolated from Secondary Lymphoid Organs
(A) Schematic view on the foxp3 locus depicts exon-intron structure and position of selected amplicons (Amp 1–4). Shown is the distribution and position of individual CpG motifs within the amplicons. (B) CD25+CD4+ and CD25−CD4+ T cells were sorted from spleens and LNs pooled from 20 male BALB/c mice. FACS analysis shows the sort purity (upper panel) and Foxp3 expression in sorted subsets (lower panel). Numbers display frequency of cells within indicated populations. The bars in the lower graphs indicate the marker gate for Foxp3+ cells. The vertical and horizontal lines in the upper graphs indicate the quadrant used to identify the CD4+CD25+/− subsets. (C) Methylation pattern of selected amplicons of the foxp3 locus in CD25+CD4+ Tregs and conventional CD25−CD4+ T cells. The amplicons are subdivided by horizontal lines each representing an individual CpG motif. ESME software [47] is used for normalization and quantification of methylation signals from sequencing data by calculating ratios of T and C signals at CpG sites. Data are condensed to methylation information at CpG positions forming matrices of consecutive CpGs. The methylation status of individual CpG motifs within the four amplicons is color coded according to the degree of methylation at that site. The color code ranges from yellow (0% methylation) to blue (100% methylation) according to the color scale on the right. CpG motifs from amplicon 2 overlapping with motifs in amplicon 1 were excluded. Due to sequencing problems, the CpG motif 37 from amplicon 4 is not listed. One representative experiment out of two individual experiments is shown.
Figure 3. Differentially Methylated, Conserved Element of the foxp3 Locus Possesses Transcriptional Activity
RLM-11–1 cells were transfected with control vector (pGL3 promoter) or Foxp3-CE vector containing the conserved element (CE). Cells were stimulated with PMA for 24 h. Control cells were left unstimulated. Results given are relative luciferase light units (RLUs) normalized for Renilla luciferase activity (mean ± standard deviation; n = 3). Results are representative for two independent experiments.
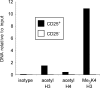
Figure 4. Increased Histone Acetylation and K4 Trimethylation in CD25+CD4+ Tregs
ChIP assays were performed with CD25+CD4+ Tregs (filled bars) and conventional CD25−CD4+ T cells (open bars) sorted from spleens and LNs pooled from 20 male BALB/c mice. DNA fragments binding to acetylated or trimethylated histones were immunoprecipitated using antibodies directed against acetylated histone H3, acetylated histone H4, or trimethylated histone H3 at position K4. A rabbit isotype immunoglobulin G (IgG) served as control. Precipitated DNA was quantified by real-time PCR with primers specific for the differentially methylated region of the foxp3 locus. Sample PCR products were set in relation to input DNA. One representative experiment out of two individual experiments is shown.
Figure 5. Demethylated CpG Motifs within the foxp3 Locus in CD25+ CD4 SP Thymocytes
(A) CD25+CD4+CD8− and CD25−CD4+CD8− subsets were sorted from thymocytes pooled from 30 male BALB/c mice. FACS analysis shows purity of sorted subsets (upper panel) and Foxp3 expression in gated CD25− and CD25+ subsets of CD4+ SP thymocytes (lower panel). Numbers display frequency of cells within indicated populations. The bars in the lower graphs indicate the marker gate for Foxp3+ cells. The vertical and horizontal lines in the upper graphs indicate the quadrant used to identify the CD4+CD25+/− subsets. (B) Methylation pattern of selected amplicons of the foxp3 locus in CD25+ and CD25− CD4 SP thymocytes. The methylation status of individual CpG motifs within the four amplicons is color coded as described in Figure 2. One representative experiment out of two individual experiments is shown.
Figure 6. TGF-β–Induced Tregs Harbor Partially Demethylated CpG Motifs within the foxp3 Locus
(A) CD25−CD4+ T cells sorted from spleens and LNs pooled from 20 male BALB/c mice were cultured for 6 d in the presence of TGF-β. Cells cultured under Th1 conditions were used as control. Within the starting population, less than 1% of the cells were Foxp3+ (unpublished data). On day 6, cultured cells were sorted by FACS for CD25+ cells. FACS analysis shows the purity of sorted subsets (upper panel) and Foxp3 expression in gated CD25+ cells derived from indicated cultures (lower panel). Numbers display frequency of cells within indicated populations. The bars in the lower graphs indicate the marker gate for Foxp3+ cells. The vertical and horizontal lines in the upper graphs indicate the quadrant used to identify the CD4+CD25+/− subsets. (B) Methylation pattern of selected amplicons of the foxp3 locus in sorted CD25+ cells derived from indicated cultures. The methylation status of individual CpG motifs within the four amplicons is color coded as described in Figure 2. One representative experiment out of two individual experiments is shown. n.a., not analyzed.
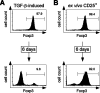
Figure 7. TGF-β–Induced Foxp3+ Tregs Loose Foxp3 Expression upon Restimulation
(A) CD25−CD4+ T cells were cultured for 6 d in the presence of TGF-β as described in Figure 6. On day 6, cultured cells were sorted by FACS for CD25+ cells and analyzed for intracellular Foxp3 expression by FACS (upper panel). Sorted CD25+ cells from the same TGF-β-cultures were restimulated for another 6 d in the absence of TGF-β. On day 6, cultured cells were analyzed for intracellular Foxp3 expression by FACS (lower panel). Numbers display frequency of cells within indicated populations. (B) Ex vivo isolated CD25+CD4+ Tregs were analyzed for intracellular Foxp3 expression before (upper panel) and after 6 d in vitro culture (lower panel) by FACS. Numbers display frequency of cells within indicated populations. One representative experiment out of two individual experiments is shown.
Similar articles
- DNA methylation controls Foxp3 gene expression.
Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. Polansky JK, et al. Eur J Immunol. 2008 Jun;38(6):1654-63. doi: 10.1002/eji.200838105. Eur J Immunol. 2008. PMID: 18493985 - Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus.
Toker A, Engelbert D, Garg G, Polansky JK, Floess S, Miyao T, Baron U, Düber S, Geffers R, Giehr P, Schallenberg S, Kretschmer K, Olek S, Walter J, Weiss S, Hori S, Hamann A, Huehn J. Toker A, et al. J Immunol. 2013 Apr 1;190(7):3180-8. doi: 10.4049/jimmunol.1203473. Epub 2013 Feb 18. J Immunol. 2013. PMID: 23420886 - Epigenetic mechanisms of regulation of Foxp3 expression.
Lal G, Bromberg JS. Lal G, et al. Blood. 2009 Oct 29;114(18):3727-35. doi: 10.1182/blood-2009-05-219584. Epub 2009 Jul 29. Blood. 2009. PMID: 19641188 Free PMC article. Review. - Induction of Foxp3 demethylation increases regulatory CD4+CD25+ T cells and prevents the occurrence of diabetes in mice.
Zheng Q, Xu Y, Liu Y, Zhang B, Li X, Guo F, Zhao Y. Zheng Q, et al. J Mol Med (Berl). 2009 Dec;87(12):1191-205. doi: 10.1007/s00109-009-0530-8. Epub 2009 Oct 20. J Mol Med (Berl). 2009. PMID: 19841877 - Induction and maintenance of regulatory T cells by transcription factors and epigenetic modifications.
Iizuka-Koga M, Nakatsukasa H, Ito M, Akanuma T, Lu Q, Yoshimura A. Iizuka-Koga M, et al. J Autoimmun. 2017 Sep;83:113-121. doi: 10.1016/j.jaut.2017.07.002. Epub 2017 Jul 11. J Autoimmun. 2017. PMID: 28709726 Review.
Cited by
- Nuclear receptor corepressor 1 controls regulatory T cell subset differentiation and effector function.
Stolz V, de Freitas E Silva R, Rica R, Zhu C, Preglej T, Hamminger P, Hainberger D, Alteneder M, Müller L, Waldherr M, Waltenberger D, Hladik A, Agerer B, Schuster M, Frey T, Krausgruber T, Knapp S, Campbell C, Schmetterer K, Trauner M, Bergthaler A, Bock C, Boucheron N, Ellmeier W. Stolz V, et al. Elife. 2024 Oct 28;13:e78738. doi: 10.7554/eLife.78738. Elife. 2024. PMID: 39466314 Free PMC article. - Transcriptional re-programming of liver-resident iNKT cells into T-regulatory type-1-like liver iNKT cells involves extensive gene de-methylation.
Montaño J, Garnica J, Yamanouchi J, Moro J, Solé P, Mondal D, Serra P, Yang Y, Santamaria P. Montaño J, et al. Front Immunol. 2024 Sep 9;15:1454314. doi: 10.3389/fimmu.2024.1454314. eCollection 2024. Front Immunol. 2024. PMID: 39315110 Free PMC article. - T-regulatory cells require Sin3a for stable expression of Foxp3.
Christensen LM, Akimova T, Wang L, Han R, Samanta A, Di Giorgio E, Hancock WW. Christensen LM, et al. Front Immunol. 2024 Aug 2;15:1444937. doi: 10.3389/fimmu.2024.1444937. eCollection 2024. Front Immunol. 2024. PMID: 39156895 Free PMC article. - Inferring upstream regulatory genes of FOXP3 in human regulatory T cells from time-series transcriptomic data.
Magni S, Sawlekar R, Capelle CM, Tslaf V, Baron A, Zeng N, Mombaerts L, Yue Z, Yuan Y, Hefeng FQ, Gonçalves J. Magni S, et al. NPJ Syst Biol Appl. 2024 May 29;10(1):59. doi: 10.1038/s41540-024-00387-9. NPJ Syst Biol Appl. 2024. PMID: 38811598 Free PMC article. - Targeted Therapy of Multiple Sclerosis: A Case for Antigen-Specific Tregs.
Zhong Y, Stauss HJ. Zhong Y, et al. Cells. 2024 May 8;13(10):797. doi: 10.3390/cells13100797. Cells. 2024. PMID: 38786021 Free PMC article. Review.
References
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. - PubMed
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. - PubMed
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. - PubMed
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials