Depolarization and CaM kinase IV modulate NMDA receptor splicing through two essential RNA elements - PubMed (original) (raw)
Depolarization and CaM kinase IV modulate NMDA receptor splicing through two essential RNA elements
Ji-Ann Lee et al. PLoS Biol. 2007 Feb.
Abstract
Alternative splicing controls the activity of many proteins important for neuronal excitation, but the signal-transduction pathways that affect spliced isoform expression are not well understood. One particularly interesting system of alternative splicing is exon 21 (E21) of the NMDA receptor 1 (NMDAR1 E21), which controls the trafficking of NMDA receptors to the plasma membrane and is repressed by Ca(++)/calmodulin-dependent protein kinase (CaMK) IV signaling. Here, we characterize the splicing of NMDAR1 E21. We find that E21 splicing is reversibly repressed by neuronal depolarization, and we identify two RNA elements within the exon that function together to mediate the inducible repression. One of these exonic elements is similar to an intronic CaMK IV-responsive RNA element (CaRRE) originally identified in the 3' splice site of the BK channel STREX exon, but not previously observed within an exon. The other element is a new RNA motif. Introduction of either of these two motifs, called CaRRE type 1 and CaRRE type 2, into a heterologous constitutive exon can confer CaMK IV-dependent repression on the new exon. Thus, either exonic CaRRE can be sufficient for CaMK IV-induced repression. Single nucleotide scanning mutagenesis defined consensus sequences for these two CaRRE motifs. A genome-wide motif search and subsequent RT-PCR validation identified a group of depolarization-regulated alternative exons carrying CaRRE consensus sequences. Many of these exons are likely to alter neuronal function. Thus, these two RNA elements define a group of co-regulated splicing events that respond to a common stimulus in neurons to alter their activity.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
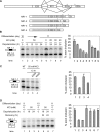
Figure 1. Depolarization Induces Splicing Repression of NMDAR1 Exon 21 in Differentiated P19 Cells
(A) Top: diagram of the alternative splicing at the 3′ end of NMDAR1 pre-mRNA. Exon 21 is an alternative exon that can be spliced to either exon 22a or 22b through the use of alternative 3′ splice sites. Asterisks (*) indicate the stop codons in E22a and E22b. Bottom: the four isoforms of the spliced mRNA. The protein-coding region is depicted as a gray bar in the mRNA. (B) Depolarization represses the splicing of E21 to E22a in differentiated P19 cells. Left panel: denaturing gel electrophoresis of the NR1–1 and NR1–2 RT-PCR products from day 10 differentiated P19 cells depolarized with 50 mM KCl for 0, 6, 12, 18, or 24 h (lanes 1–5), from day 11 untreated cells (lane 6), and from day 10 cells depolarized with 20, 30, or 40 mM KCl for 24 h. Right panel: percentage of mRNA containing E21 after depolarization ((NR1–1/(NR1–1 + NR1–2))*100). Bars indicate mean ± standard deviation (s.d.), n = 3. (C) Depolarization-induced repression is dependent on CaMK. Left panel: RT-PCR assay of NR1–1 and NR1–2 mRNA from differentiated P19 cells treated with KCl, KCl plus KN93, or KCl plus KN92 for 12 h. Right panel: graph of E21 inclusion as shown in the left panel. (D) Splicing repression is reversible after removal of the stimulus. Left: RT-PCR assay of NR1–1 and NR1–2 mRNA isolated from cells depolarized with 50 mM KCl for 18 or 24 h and 24 hours after the salt washout. Right: graph of E21 inclusion as shown in the left panel.
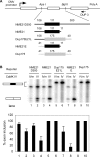
Figure 2. The Exonic Sequence of NR1 E21 Is Sufficient for CaMK IV–Induced Splicing Repression in HEK 293T Cells
(A) Top: diagram of the pDup minigene reporter. The reporter has a three-exon structure, and the second exon can be replaced with other exons between ApaI and BglII sites in the introns. The CMV promoter, exons (boxes), introns (lines), and primer extension primer DUP 3 (arrow) are indicated. Bottom: structures of hybrid second exons. Sequences derived from human NR1 E21 are depicted as black lines and boxes; sequences derived from _β_-globin sequences of the second exon in Dup175 construct are depicted as grey lines and boxes. The lengths of these exons and intron segments are indicated above the exon diagram, and names of these reporters are indicated to the left of the exon diagram. (B) Top: primer extension assay of reporters co-expressed with CaMK IV-dCTK75E (IVm), or CaMK IV-dCT (IV). Bottom: graph of exon inclusion from the gel above.
Figure 3. Exon Scanning and Point Mutagenesis Reveal Sequences Needed for CaMK IV–Induced Splicing Repression
(A) The exonic sequence and splice sites of human E21. The replacement of 20 nucleotides of exon sequence with the IgM sequence UCAGCAUGACUCUCAGCAUG is indicated by a line under the replaced sequence, and the clone name is below the line. LS7 carried only a 16-nucleotide exon replacement with the first 16 nucleotides of the IgM sequence. Specific mutations are indicated by bold type above the nucleotides, and the clone name is above the mutation. The R1 mutation is generated by replacing the boxed sequence with the IgM sequence CAUGACUCUCAG. The predicted sites of the CaRRE 1 and the hnRNP A1 binding site are italicized. (B) Primer extension assay of the LS mutants co-expressed with CaMK IV-dCTK75E (IVm) or CaMK IV-dCT (IV). Percentages of exon inclusion and standard deviations (n = 3) are indicated beneath the lanes. (C) Primer extension assay of specific point mutants co-expressed with CaMK IV-dCTK75E (IVm) or CaMK IV-dCT (IV). Percentages of exon inclusion and standard deviations (n = 3) are indicated beneath the lanes.
Figure 4. Either Exonic CaRRE Is Sufficient for CaMK IV Repression in a Heterologous Exon
(A) Right: diagram of the chimeric exons generated by replacing nucleotides 13–24 or 72–83 of exon 2 in the Dup175 minigene with the NR1 E21 CaRRE 1 sequence (NR1 E21 nucleotides 45–56, filled box), a mutant sequence (gray box), or an unrelated IgM sequence (dotted box). The clone numbers and the replacement sequences are indicated to the left and right, respectively. Left: primer extension assay of the minigenes indicated above. (B) Right: diagrams of the chimeric exons generated by inserting NR1 E21 nucleotides 3–22 (CaRRE 2, dark-hatched box), a mutant sequence (light-hatched box), or an unrelated IgM sequence into Dup175 exon at the +3 position. The clone numbers and the inserted sequences are indicated to the left and right, respectively. Left: primer extension assay of the minigenes indicated above. (C) Right: Dup175PR1CA10 and Dup175PR1CA13 exons are generated by replacing nucleotides 33–44 or 92–103, respectively, in the Dup175PR1 exon with the NR1 E21 CaRRE 1 sequence. Dup175PR1CA3, Dup175PR1MCA3, and Dup175PR1RCA3 were generated by replacing the E21 CaRRE 1 sequence with the NR1 E5 intronic CaRRE 1 sequence (gray box). Left: primer extension assay of the minigenes indicated above. The asterisk (*) indicates a cryptic splicing event.
Figure 5. Degenerate Sequences Can Act as CaRRE 1 and CaRRE 2
(A) and (B) Top: single nucleotide scanning mutagenesis of CaRRE 1 and CaRRE 2. The ApaI site of pDup175 was mutated to XhoI site to facilitate the cloning. Each nucleotide of CaRRE 1 (A) and CaRRE 2 (B) was mutated to the other three possible nucleotides, one at a time. Each mutant was co-transfected with CaMK IV-dCTK75E (m) or CaMK IV-dCT (IV) Bottom right of (A) and (B): summary of the relative activity of the CaRRE mutants. The activities of wild-type CaRREs were defined as 100%, and the relative activity of each mutant was calculated by normalizing the difference of the percentage of exon inclusion caused by CaMK IVdCT to the difference observed in its wild-type CaRRE. These mutants are classified by their relative activities (left column). The 8A to 8C mutation in CaRRE 2 disrupted the splicing of this minigene, and the relative exon inclusion could not be determined.
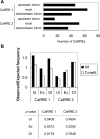
Figure 6. Genome-Wide Identification of Alternative Exons with CaRRE 1 and CaRRE 2 Sequences
(A) A database of 2,461 alternatively spliced exons was searched for the family of degenerate CaRRE 1 and CaRRE 2 sequences. The search included the exon and 50 nucleotides of upstream and downstream intron sequences. The number of CaRREs found in these different regions is illustrated. (B) CaRRE motif frequencies. The CaRRE 1 and CaRRE 2 motif frequencies in the upstream intron (UI; position −50 to −1), the exon (Ex), and the downstream intron (DI; position 1 to 50) were calculated separately from a dataset of 2,461 alternative exons (Alt) and a dataset of 9,401 constitutive exons (Constit). The observed CaRRE frequency was calculated by dividing the number of CaRREs by the number of octamers in the searched region. This observed frequency was then normalized to the expected frequency of the random octamers. The _p_-values are given below the histogram.
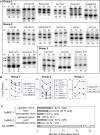
Figure 7. RT-PCR Examination of Depolarization-Induced Splicing Changes of CaRRE-Containing Exons in Differentiated P19 Cells
(A) Regulated alternative exons. Sixteen CaRRE-containing exons and one control alternative exon showed splicing changes in the semi-quantitive RT-PCR analysis (22–25 cycles). Each gel panel shows the splicing patterns in rested cells (0 h) and cells treated with 50 mM KCl for 12 and 24 h. These exons are classified by their responses to depolarization. Group 1 contains exons whose splicing was repressed through 24 h. Group 2 contains exons whose splicing was repressed at 12 h, but recovered at 24 h. Group 3 contains exons whose splicing was enhanced by depolarization. The use of alternative 5′ splice sites in Atp2b1 E21 generates two isoforms that are indicated by a and c, and the exon-skipped isoform is indicated by b to the left of the gel. (B) Graphs of exon inclusion as shown above. (C) Summary of the semi-quantitative RT-PCR for the selected alternative exons. The regulated alternative exons are depicted as a gray bar and the non-regulated alternative exons are depicted as an open bar. The percentages of regulated alternative exons among the tested alternative exons in each category are indicated to the right of the bars, and the numbers of regulated and tested alternative exons are shown in the parentheses. More information is summarized in Table 1 and Table S1.
Figure 8. Mutagenesis Analysis Confirms the Activity of CaRRE 1 in the Regulated Exons
Left: exons and the flanking introns were cloned from the mouse genomic DNA into pDup4–1. The length (in nucleotides) of each region is indicated above the diagram. The replaced and deleted nucleotides (dashed bar) in each mutant are shown below the wild-type sequence. Right: primer extension assay of the mutants indicated above.
Comment in
- Sing the genome electric: excited cells adjust their splicing.
Ares M Jr. Ares M Jr. PLoS Biol. 2007 Feb;5(2):e55. doi: 10.1371/journal.pbio.0050055. PLoS Biol. 2007. PMID: 17298182 Free PMC article.
Similar articles
- A CaMK IV responsive RNA element mediates depolarization-induced alternative splicing of ion channels.
Xie J, Black DL. Xie J, et al. Nature. 2001 Apr 19;410(6831):936-9. doi: 10.1038/35073593. Nature. 2001. PMID: 11309619 - A consensus CaMK IV-responsive RNA sequence mediates regulation of alternative exons in neurons.
Xie J, Jan C, Stoilov P, Park J, Black DL. Xie J, et al. RNA. 2005 Dec;11(12):1825-34. doi: 10.1261/rna.2171205. RNA. 2005. PMID: 16314456 Free PMC article. - Exon silencing by UAGG motifs in response to neuronal excitation.
An P, Grabowski PJ. An P, et al. PLoS Biol. 2007 Feb;5(2):e36. doi: 10.1371/journal.pbio.0050036. PLoS Biol. 2007. PMID: 17298175 Free PMC article. - Neuronal signaling through alternative splicing: some exons CaRRE.
O'Donovan KJ, Darnell RB. O'Donovan KJ, et al. Sci STKE. 2001 Aug 7;2001(94):pe2. doi: 10.1126/stke.2001.94.pe2. Sci STKE. 2001. PMID: 11752670 Review. - The neuronal splicing factor Nova controls alternative splicing in N-type and P-type CaV2 calcium channels.
Allen SE, Darnell RB, Lipscombe D. Allen SE, et al. Channels (Austin). 2010 Nov-Dec;4(6):483-9. doi: 10.4161/chan.4.6.12868. Epub 2010 Nov 1. Channels (Austin). 2010. PMID: 21150296 Free PMC article. Review.
Cited by
- Computational characterization of 3' splice variants in the GFAP isoform family.
Boyd SE, Nair B, Ng SW, Keith JM, Orian JM. Boyd SE, et al. PLoS One. 2012;7(3):e33565. doi: 10.1371/journal.pone.0033565. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479412 Free PMC article. - Ca2+-signaling, alternative splicing and endoplasmic reticulum stress responses.
Krebs J, Groenendyk J, Michalak M. Krebs J, et al. Neurochem Res. 2011 Jul;36(7):1198-211. doi: 10.1007/s11064-011-0431-4. Epub 2011 Mar 2. Neurochem Res. 2011. PMID: 21365449 Review. - Signal- and development-dependent alternative splicing of LEF1 in T cells is controlled by CELF2.
Mallory MJ, Jackson J, Weber B, Chi A, Heyd F, Lynch KW. Mallory MJ, et al. Mol Cell Biol. 2011 Jun;31(11):2184-95. doi: 10.1128/MCB.05170-11. Epub 2011 Mar 28. Mol Cell Biol. 2011. PMID: 21444716 Free PMC article. - Phenylbutazone induces expression of MBNL1 and suppresses formation of MBNL1-CUG RNA foci in a mouse model of myotonic dystrophy.
Chen G, Masuda A, Konishi H, Ohkawara B, Ito M, Kinoshita M, Kiyama H, Matsuura T, Ohno K. Chen G, et al. Sci Rep. 2016 Apr 29;6:25317. doi: 10.1038/srep25317. Sci Rep. 2016. PMID: 27126921 Free PMC article. - A conserved serine of heterogeneous nuclear ribonucleoprotein L (hnRNP L) mediates depolarization-regulated alternative splicing of potassium channels.
Liu G, Razanau A, Hai Y, Yu J, Sohail M, Lobo VG, Chu J, Kung SK, Xie J. Liu G, et al. J Biol Chem. 2012 Jun 29;287(27):22709-16. doi: 10.1074/jbc.M112.357343. Epub 2012 May 8. J Biol Chem. 2012. PMID: 22570490 Free PMC article.
References
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: Implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. - PubMed
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. - PubMed
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, et al. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. - PubMed
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials