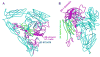Tests of the extension and deadbolt models of integrin activation - PubMed (original) (raw)
Tests of the extension and deadbolt models of integrin activation
Jieqing Zhu et al. J Biol Chem. 2007.
Abstract
Despite extensive evidence that integrin conformational changes between bent and extended conformations regulate affinity for ligands, an alternative hypothesis has been proposed in which a "deadbolt" can regulate affinity for ligand in the absence of extension. Here, we tested both the deadbolt and the extension models. According to the deadbolt model, a hairpin loop in the beta3 tail domain could act as a deadbolt to restrain the displacement of the beta3 I domain beta6-alpha7 loop and maintain integrin in the low affinity state. We found that mutating or deleting the beta3 tail domain loop has no effect on ligand binding by either alphaIIbbeta 3 or alphaVbeta3 integrins. In contrast, we found that mutations that lock integrins in the bent conformation with disulfide bonds resist inside-out activation induced by cytoplasmic domain mutation. Furthermore, we demonstrated that extension is required for accessibility to fibronectin but not smaller fragments. The data demonstrate that integrin extension is required for ligand binding during integrin inside-out signaling and that the deadbolt does not regulate integrin activation.
Figures
FIGURE 1. Locations of the mutations in the αVβ 3 crystal structure (3)
The _α_V subunit is in cyan. The _β_3 hybrid domain is in green, and other _β_3 domains are in magenta. The _β_3 tail domain CD loop (residues Asp-672–Lys-676) is in blue. Residues mutated to cysteine are shown with spheres at the positions of α_V G307 C_α (cyan) and β_3 R563 C_α (magenta). The position of the _N_-glycan wedge introduced by the β_3 NIN305T mutation is shown with a yellow sphere at Asn-303 C_α. A, this view emphasizes the _β_-I domain _β_6-strand and _α_7-helix, the only elements shown as a ribbon, and their proximity to the _β_-tail domain CD loop. B, this view is rotated relative to A and emphasizes the glycan wedge introduced into a crevice between the hybrid and _β_-I domain that widens in the open headpiece conformation.
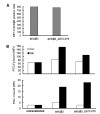
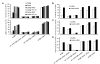
FIGURE 2. Effect of _β_-tail domain CD loop mutation on ligand binding by _α_IIb_β_3 expressed on CHO cells
A, expression of wild-type and mutant _α_IIb/_β_3 integrins in CHO cells is shown as MFI of _α_IIb/_β_3-positive cells stained with AP3-conjugated with Alexa Fluor 647. B, mock transfectants or _α_IIb/_β_3-positive cells gated for equivalent _β_3 expression using AP3 mAb were quantitated for binding of FITC-Fg or the fibrinogen mimetic antibody, PAC-1, in the presence of 1 mM Ca2+ or 1 mM Mn2+.
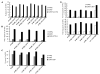
FIGURE 3. Effect of _β_-tail domain CD loop mutation on ligand binding by _α_IIb_β_3 and _α_V_β_3 expressed in 293T cells
A, human Fg binding to _α_IIb_β_3 and _α_V_β_3. B, ligand-mimetic PAC-1 antibody binding to _α_IIb_β_3. C, human Fn binding to _α_V_β_3. D, binding of fibronectin fragments Fn9–10 and Fn7–10 to _α_V_β_3. 293T cell transfectants were treated with the indicated conditions and incubated with 50 _μ_g/ml fluorescently labeled ligands or 10 _μ_g/ml PAC-1 IgM as described under “Experimental Procedures.” Binding is expressed as MFI of ligand staining as a percentage of MFI of Cy3-AP3 antibody staining.
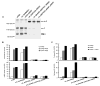
FIGURE 4. Intersubunit disulfide bond formation and effect on LIBS exposure and ligand binding
A, disulfide bond formation by cysteine mutants. Lysates from 35S-labeled 293T cells that had been transiently transfected with wild-type or mutant integrins as indicated were immunoprecipitated with mAb AP3 (anti-_β_3) and subjected to non-reducing SDS-7.5% PAGE. Bands of _α_V (α), _β_3 (β), and _α_V_β_3 heterodimer (α-β) are indicated. Positions of protein molecular size markers are shown on the left. B, LIBS exposure. 293T cell transfectants were stained with anti-LIBS mAb D3 or LIBS1 in the presence of 1 mM Ca2+/1 mM Mg2+ or 1 mM Ca2+/1 mM Mn2+, with or without 25 _μ_M cyclo-RGDfV (RGD). LIBS epitope expression is expressed as MFI of the D3 or LIBS1 staining as a percentage of MFI of AP3 mAb staining. C, soluble Fn or Fg binding to 293T cell transfectants in the presence of 1 mM Ca2+/1 mM Mg2+ or 1 mM Ca2+/1 mM Mn2+ or 1 mM Ca2+/1 mM Mn2+ plus 10 _μ_g/ml activating mAb, LIBS1. Binding is expressed as MFI of ligand staining as a percentage of MFI of staining with Cy3-AP3 antibody.
FIGURE 5. LIBS exposure and ligand binding of disulfide-bonded and glycan wedge mutants
A, LIBS exposure. 293T cell transfectants were stained with anti-LIBS antibodies, D3 or LIBS1, in the presence of 1 mM Ca2+/1 mM Mg2+ or 0.2 mM Ca2+/0.2 mM Mn2+, with or without 25 _μ_M cyclo-RGDfV or 1 mg/ml Fn7–10. LIBS epitope expression is expressed as MFI of the D3 or LIBS1 staining as a percentage of MFI of AP3 mAb staining. B, Fn binding. C, fibronectin fragment Fn7–10 binding. D, fibronectin fragment Fn9–10 binding. In B–D, 293T cell transfectants were stained with 50 _μ_g/ml fluorescently labeled ligands in the presence of 1 mM Ca2+/1 mM Mg2+ or 1 mM Ca2+/1 mM Mn2+ plus 10 _μ_g/ml activating mAb, LIBS1, and subjected to flow cytometry. Binding is expressed as MFI of ligand staining as a percentage of MFI of Cy3-AP3 antibody staining.
Similar articles
- Integrin αII b tail distal of GFFKR participates in inside-out αII b β3 activation.
Li A, Guo Q, Kim C, Hu W, Ye F. Li A, et al. J Thromb Haemost. 2014 Jul;12(7):1145-55. doi: 10.1111/jth.12610. Epub 2014 Jun 25. J Thromb Haemost. 2014. PMID: 24837519 Free PMC article. - Identification of interacting hot spots in the beta3 integrin stalk using comprehensive interface design.
Donald JE, Zhu H, Litvinov RI, DeGrado WF, Bennett JS. Donald JE, et al. J Biol Chem. 2010 Dec 3;285(49):38658-65. doi: 10.1074/jbc.M110.170670. Epub 2010 Oct 7. J Biol Chem. 2010. PMID: 20929856 Free PMC article. - Cooperative role of the membrane-proximal and -distal residues of the integrin beta3 cytoplasmic domain in regulation of talin-mediated alpha IIb beta3 activation.
Hato T, Yamanouchi J, Tamura T, Yakushijin Y, Sakai I, Yasukawa M. Hato T, et al. J Biol Chem. 2008 Feb 29;283(9):5662-8. doi: 10.1074/jbc.M707246200. Epub 2008 Jan 2. J Biol Chem. 2008. PMID: 18174155 - Atypical structure and function of integrin αV β8.
Song G, Luo BH. Song G, et al. J Cell Physiol. 2021 Jul;236(7):4874-4887. doi: 10.1002/jcp.30242. Epub 2020 Dec 27. J Cell Physiol. 2021. PMID: 33368230 Review. - The beta3 integrin cytoplasmic tail: protein scaffold and control freak.
Shattil SJ. Shattil SJ. J Thromb Haemost. 2009 Jul;7 Suppl 1:210-3. doi: 10.1111/j.1538-7836.2009.03397.x. J Thromb Haemost. 2009. PMID: 19630802 Review.
Cited by
- Data-driven prediction of αIIbβ3 integrin activation paths using manifold learning and deep generative modeling.
Dasetty S, Bidone TC, Ferguson AL. Dasetty S, et al. Biophys J. 2024 Sep 3;123(17):2716-2729. doi: 10.1016/j.bpj.2023.12.009. Epub 2023 Dec 14. Biophys J. 2024. PMID: 38098231 - Cryo-EM structures of full-length integrin αIIbβ3 in native lipids.
Adair BD, Xiong JP, Yeager M, Arnaout MA. Adair BD, et al. Nat Commun. 2023 Jul 13;14(1):4168. doi: 10.1038/s41467-023-39763-0. Nat Commun. 2023. PMID: 37443315 Free PMC article. - β2 integrin activation and signal transduction in leukocyte recruitment.
Sun H, Hu L, Fan Z. Sun H, et al. Am J Physiol Cell Physiol. 2021 Aug 1;321(2):C308-C316. doi: 10.1152/ajpcell.00560.2020. Epub 2021 Jun 16. Am J Physiol Cell Physiol. 2021. PMID: 34133240 Free PMC article. Review. - Modulating Integrin αIIbβ3 Activity through Mutagenesis of Allosterically Regulated Intersubunit Contacts.
Tan SK, Fong KP, Polizzi NF, Sternisha A, Slusky JSG, Yoon K, DeGrado WF, Bennett JS. Tan SK, et al. Biochemistry. 2019 Jul 30;58(30):3251-3259. doi: 10.1021/acs.biochem.9b00430. Epub 2019 Jul 12. Biochemistry. 2019. PMID: 31264850 Free PMC article. - Neutrophil Recruitment: From Model Systems to Tissue-Specific Patterns.
Margraf A, Ley K, Zarbock A. Margraf A, et al. Trends Immunol. 2019 Jul;40(7):613-634. doi: 10.1016/j.it.2019.04.010. Epub 2019 Jun 4. Trends Immunol. 2019. PMID: 31175062 Free PMC article. Review.
References
- Hynes RO. Cell. 2002;110:673–687. - PubMed
- Shattil SJ, Newman PJ. Blood. 2004;104:1606–1615. - PubMed
- Takagi J, Petre BM, Walz T, Springer TA. Cell. 2002;110:599–611. - PubMed
- Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA. Immunity. 2006;25:583–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources