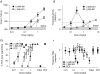Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block - PubMed (original) (raw)
Comparative Study
. 2007 May 15;581(Pt 1):107-28.
doi: 10.1113/jphysiol.2006.124958. Epub 2007 Feb 15.
Kevin Erreger, Hongjie Yuan, Katherine Nicholson, Phuong Le, Polina Lyuboslavsky, Antoine Almonte, Ernest Murray, Cara Mosely, Jeremy Barber, Adam French, Robert Balster, Thomas F Murray, Stephen F Traynelis
Affiliations
- PMID: 17303642
- PMCID: PMC2075223
- DOI: 10.1113/jphysiol.2006.124958
Comparative Study
Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block
Shashank M Dravid et al. J Physiol. 2007.
Abstract
We have compared the potencies of structurally distinct channel blockers at recombinant NR1/NR2A, NR1/NR2B, NR1/NR2C and NR1/NR2D receptors. The IC50 values varied with stereochemistry and subunit composition, suggesting that it may be possible to design subunit-selective channel blockers. For dizocilpine (MK-801), the differential potency of MK-801 stereoisomers determined at recombinant NMDA receptors was confirmed at native receptors in vitro and in vivo. Since the proton sensor is tightly linked both structurally and functionally to channel gating, we examined whether blocking molecules that interact in the channel pore with the gating machinery can differentially sense protonation of the receptor. Blockers capable of remaining trapped in the pore during agonist unbinding showed the strongest dependence on extracellular pH, appearing more potent at acidic pH values that promote channel closure. Determination of pK(a) values for channel blockers suggests that the ionization of ketamine but not of other blockers can influence its pH-dependent potency. Kinetic modelling and single channel studies suggest that the pH-dependent block of NR1/NR2A by (-)MK-801 but not (+)MK-801 reflects an increase in the MK-801 association rate even though protons reduce channel open probability and thus MK-801 access to its binding site. Allosteric modulators that alter pH sensitivity alter the potency of MK-801, supporting the interpretation that the pH sensitivity of MK-801 binding reflects the changes at the proton sensor rather than a secondary effect of pH. These data suggest a tight coupling between the proton sensor and the ion channel gate as well as unique subunit-specific mechanisms of channel block.
Figures
Figure 1
Structures of NMDA antagonists tested for their pH sensitivity
Figure 2. (+)MK-801 isomer is more potent isomer than (−)MK-801
A, the delay to the initiation of the tonic hindlimb extension (THE) is shown for C57Bl/6 mice in a chamber infused with the convulsant anaesthetic flurothyl (see Methods). The grey lines indicate the dose required to delay progression to hindlimb extension by 3 min (180 s). The filled in area at the bottom of the graph shows saline control. Data points are means ±
s.e.m.
of 8–11 rats. B, administration of MK-801 was performed after a 1 h habituation, and locomotor activity monitored over a 2 h period. The total horizontal activity from different rats was averaged and expressed as a function of dose. The biphasic nature of the response to (+)MK-801 reflected the severe ataxia that occurred with the highest doses. Data points are means ±
s.e.m.
of 3–11 rats. Ca shows the percentage of the correct PCP-lever responding and Cb the response rates. Values above PCP and saline are the results of control tests conducted before each dose–response curve. Mean percentage PCP-lever responding was based on 6 of 7 rats for the (−)MK-801 doses of 0.17 and 0.25 mg kg−1 and 3 of 7 rats for the PCP dose of 8 mg kg−1. All other data points are the means ±
s.e.m.
of 7 rats. Concentration values are plotted on a logarithmic scale.
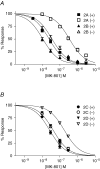
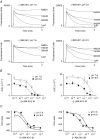
Figure 3. NR2 subunit dependence of MK-801 potency
Recombinant NMDA receptors expressed in oocytes were recorded using two-electrode voltage clamp and activated by coapplication of 50 μ
m
glutamate and 30 μ
m
glycine plus increasing concentrations of (+)MK-801 or (−)MK-801 at pH 7.6 to establish the concentration–response curve for MK-801 inhibition of all heterodimeric NR1/NR2 subunit combinations (A, NR2A or NR2B; B, NR2C or NR2D). MK-801 was applied for 1–2 min at each concentration. The oocytes were held at −40 mV. Each point represents measurements from 6–40 oocyte recordings.
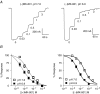
Figure 4. Differential coupling of MK-801 stereoisomers to proton-sensitive gating of recombinant NMDA receptors
A, two-electrode voltage-clamp current recordings show the inhibition of NR1/NR2A NMDA receptors by (−)MK-801 at pH 7.6 and 6.9 in Xenopus oocytes. The NMDA receptors were activated by 50 μ
m
glutamate and 30 μ
m
glycine; increasing concentrations (n
m
) of (−)MK-801 were coapplied with agonists to establish the concentration–response curve. The oocytes were held at −40 mV. B, concentration–response curves for NMDA receptor current inhibition by MK-801 isomers at pH 7.6 and 6.9 at NR1/NR2A receptors. Each point represents the mean ±
s.e.m.
of 6–40 oocyte recordings. The data for pH 7.6 are the same as those shown in Fig. 3.
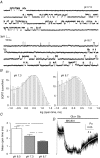
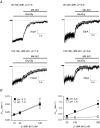
Figure 8. Non-linear fitting kinetic scheme to response waveforms suggests that (−)MK-801 has a faster association rate at protonated NR1/NR2A receptors
A, unitary currents shown were recorded from cell-attached patches on HEK 293 cells expressing NR1/NR2A receptors (filtered at 5 kHz, −3 dB). The pipette contained 1 m
m
glutamate and 50 μ
m
glycine (pipette potential +60 mV). Upward deflections reflect channel openings. Representative traces are shown for two different patches at pH values of 7.3 and 6.7. B, the distribution of open channel dwell times for the same patches shown in A is plotted as a histogram on a log-square root (SQRT) scale. The open time distribution was fitted with the sum of two exponential components for each patch (see Table 6 for mean open time constants). C, the arithmetic mean channel open duration is significantly shorter at lower pH (n = 6–9, ANOVA *P < 0.05 **P < 0.01; see Table 6 for fitted time constants). D, whole-cell recording from NR1/NR2A receptors at −60 mV (pH 6.9) in response to 50 μ
m
glutamate and 50 μ
m
glycine in the absence or presence of 100 n
m
(−)MK-801. The trace has been converted to open probability as described in the text. Data were fitted to the model depicted in Fig. 7 using a simplex algorithm to alter model parameters at each iteration; least-squares criteria were employed as determined from normalized waveforms. Fitted waveform is shown in terms of open probability predicted by the model (grey curve). The mean rate constants obtained by fitting each whole-cell recording are given in Table 7.
Figure 7. Hypothetical scheme for MK-801 binding to NR1/NR2A receptors
A model was adapted from Blanpied et al. (1997) to explain the mechanism of trapping blockers such as MK-801. R, receptor; A, agonist; B, channel blocker; D, desensitized state.
Figure 5. Differential coupling of MK-801 stereoisomers to proton-sensitive gating of native NMDA receptors
A, inhibition of NMDA (30 μ
m
; 100 μ
m
glycine)-mediated calcium influx in cerebral cortical neurons by MK-801 isomers was determined as a function of pH using the Ca2+-sensitive dye fluo-3 AM (see Methods). B, each point on the concentration–response curve for MK-801 inhibition is the mean ±
s.e.m.
of the area under the curve (n = 4–6). C, the potency of MK-801 isomers was determined against an excitotoxic insult by 30 μ
m
NMDA plus 100 μ
m
glycine in neocortical neurons (see Methods). Each point on the concentration–response curve represents the mean ±
s.e.m.
of measurements (n = 3).
Figure 6. Low pH accelerates macroscopically derived (−)MK–801 association rate with NR1/NR2A receptors
A, the time course of MK-801 (30–100 n
m
) block of whole-cell currents in HEK 293 cells expressing NR1/NR2A receptors in response to rapid application of 50 μ
m
glutamate and 50 μ
m
glycine is shown at different pH values. HEK 293 cells were held under voltage clamp at −60 mV. B, values for τON were determined by fitting the decay in current in the presence of MK-801 with an exponential function using a simplex algorithm (least-squares criteria): Response = Amplitude exp(−time/τ) + steady state. The reciprocal of τON was plotted against concentration of MK-801 to estimate macroscopically derived _k_ON and _k_OFF by: 1/τON =_k_ON[MK-801]+_k_OFF. Each point represents measurements from 3–5 cells.
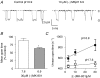
Figure 9. Single channel analysis suggests that (−)MK-801 has a faster association rate at protonated NR1/NR2A receptors
A, unitary currents recorded from outside-out patches excised from HEK 293 cells expressing NR1/NR2A receptors are shown (holding potential was −60 mV). Traces were filtered at 2 kHz for the purpose of illustration. Dotted line indicates closed state (C); open state is indicated by O. (−)MK-801 at 10 μ
m
reduces the arithmetic mean channel open duration at pH 6.9. B, mean open duration in the presence of 30 μ
m
(−)MK-801 at two different pH conditions is expressed as a percentage of mean open duration of control. Reduction in mean open duration at pH 6.9 is significantly different from at pH 7.6 (*P < 0.05 Student's unpaired t test, n = 4–5 for each condition). C, reciprocal of mean open time is plotted as a function of (−)MK-801 concentration. Each point is the mean ±
s.e.m.
determined from 4–7 patches. The slope of this relationship was determined by linear regression and represents the blocking rate of (−)MK-801. Blocking rate at pH 7.6 is 1.6 × 106
m
−1 s−1 and at pH 6.9 is 8.6 × 106
m
−1 s−1.
Comment in
- NMDA receptor antagonists: tools in neuroscience with promise for treating CNS pathologies.
Köhr G. Köhr G. J Physiol. 2007 May 15;581(Pt 1):1-2. doi: 10.1113/jphysiol.2007.130732. Epub 2007 Mar 1. J Physiol. 2007. PMID: 17331982 Free PMC article. No abstract available.
Similar articles
- Conserved structural and functional control of N-methyl-D-aspartate receptor gating by transmembrane domain M3.
Yuan H, Erreger K, Dravid SM, Traynelis SF. Yuan H, et al. J Biol Chem. 2005 Aug 19;280(33):29708-16. doi: 10.1074/jbc.M414215200. Epub 2005 Jun 21. J Biol Chem. 2005. PMID: 15970596 - NR1 and NR2 subunit contributions to N-methyl-D-aspartate receptor channel blocker pharmacology.
Monaghan DT, Larsen H. Monaghan DT, et al. J Pharmacol Exp Ther. 1997 Feb;280(2):614-20. J Pharmacol Exp Ther. 1997. PMID: 9023271 - Effects of memantine on recombinant rat NMDA receptors expressed in HEK 293 cells.
Bresink I, Benke TA, Collett VJ, Seal AJ, Parsons CG, Henley JM, Collingridge GL. Bresink I, et al. Br J Pharmacol. 1996 Sep;119(2):195-204. doi: 10.1111/j.1476-5381.1996.tb15971.x. Br J Pharmacol. 1996. PMID: 8886398 Free PMC article. - Open-channel blockers of the NMDA receptor complex.
Albensi BC, Ilkanich E. Albensi BC, et al. Drug News Perspect. 2004 Nov;17(9):557-62. doi: 10.1358/dnp.2004.17.9.872569. Drug News Perspect. 2004. PMID: 15645013 Review. - Subunit- and site-specific pharmacology of the NMDA receptor channel.
Yamakura T, Shimoji K. Yamakura T, et al. Prog Neurobiol. 1999 Oct;59(3):279-98. doi: 10.1016/s0301-0082(99)00007-6. Prog Neurobiol. 1999. PMID: 10465381 Review.
Cited by
- Specific sites within the ligand-binding domain and ion channel linkers modulate NMDA receptor gating.
Talukder I, Borker P, Wollmuth LP. Talukder I, et al. J Neurosci. 2010 Sep 1;30(35):11792-804. doi: 10.1523/JNEUROSCI.5382-09.2010. J Neurosci. 2010. PMID: 20810899 Free PMC article. - NMDARs mediate peripheral and central sensitization contributing to chronic orofacial pain.
Liu YJ, Li YL, Fang ZH, Liao HL, Zhang YY, Lin J, Liu F, Shen JF. Liu YJ, et al. Front Cell Neurosci. 2022 Sep 27;16:999509. doi: 10.3389/fncel.2022.999509. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36238833 Free PMC article. Review. - Ketamine: NMDA Receptors and Beyond.
Zorumski CF, Izumi Y, Mennerick S. Zorumski CF, et al. J Neurosci. 2016 Nov 2;36(44):11158-11164. doi: 10.1523/JNEUROSCI.1547-16.2016. J Neurosci. 2016. PMID: 27807158 Free PMC article. - Inhibition of NMDA receptors through a membrane-to-channel path.
Wilcox MR, Nigam A, Glasgow NG, Narangoda C, Phillips MB, Patel DS, Mesbahi-Vasey S, Turcu AL, Vázquez S, Kurnikova MG, Johnson JW. Wilcox MR, et al. Nat Commun. 2022 Jul 15;13(1):4114. doi: 10.1038/s41467-022-31817-z. Nat Commun. 2022. PMID: 35840593 Free PMC article. - The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice.
Kos T, Nikiforuk A, Rafa D, Popik P. Kos T, et al. Psychopharmacology (Berl). 2011 Apr;214(4):911-21. doi: 10.1007/s00213-010-2102-6. Epub 2010 Dec 16. Psychopharmacology (Berl). 2011. PMID: 21161188 Free PMC article.
References
- Albers GW, Goldberg MP, Choi DW. N-Methyl-d-aspartate antagonists: ready for clinical trial in brain ischemia? Ann Neurol. 1989;25:398–403. - PubMed
- Andine P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Martensson E, Sandberg M. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. - PubMed
- Antonov SM, Gmiro VE, Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nat Neurosci. 1998;1:451–461. - PubMed
- Balster RL. Drug abuse potential evaluation in animals. Br J Addict. 1991;86:1549–1558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA001442-30/DA/NIDA NIH HHS/United States
- R01 DA001442/DA/NIDA NIH HHS/United States
- R01-NS36777/NS/NINDS NIH HHS/United States
- DA-07218/DA/NIDA NIH HHS/United States
- R01-01442/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases