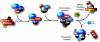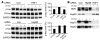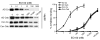The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins - PubMed (original) (raw)
. 2007 Mar;117(3):648-58.
doi: 10.1172/JCI29715. Epub 2007 Feb 15.
Adeela Kamal, Karen Lundgren, Natalia Klosak, Rachel M Bailey, Judith Dunmore, Peter Ash, Sareh Shoraka, Jelena Zlatkovic, Christopher B Eckman, Cam Patterson, Dennis W Dickson, N Stanley Nahman Jr, Michael Hutton, Francis Burrows, Leonard Petrucelli
Affiliations
- PMID: 17304350
- PMCID: PMC1794119
- DOI: 10.1172/JCI29715
The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins
Chad A Dickey et al. J Clin Invest. 2007 Mar.
Abstract
A primary pathologic component of Alzheimer's disease (AD) is the formation of neurofibrillary tangles composed of hyperphosphorylated tau (p-tau). Expediting the removal of these p-tau species may be a relevant therapeutic strategy. Here we report that inhibition of Hsp90 led to decreases in p-tau levels independent of heat shock factor 1 (HSF1) activation. A critical mediator of this mechanism was carboxy terminus of Hsp70-interacting protein (CHIP), a tau ubiquitin ligase. Cochaperones were also involved in Hsp90-mediated removal of p-tau, while those of the mature Hsp90 refolding complex prevented this effect. This is the first demonstration to our knowledge that blockade of the refolding pathway promotes p-tau turnover through degradation. We also show that peripheral administration of a novel Hsp90 inhibitor promoted selective decreases in p-tau species in a mouse model of tauopathy, further suggesting a central role for the Hsp90 complex in the pathogenesis of tauopathies. When taken in the context of known high-affinity Hsp90 complexes in affected regions of the AD brain, these data implicate a central role for Hsp90 in the development of AD and other tauopathies and may provide a rationale for the development of novel Hsp90-based therapeutic strategies.
Figures
Figure 1. EC102 crosses the blood-brain barrier and reduces tau levels in cells after 24 hours.
(A) CD-1 mice were injected i.p. with the indicated doses of EC102 and harvested 1, 3, 6, and 24 hours after injection. Brain levels of EC102 were assessed by HPLC analysis. Greater than 50% concentration was maintained for 3 hours with 200 mg/kg without detectable toxicity. (B) CD-1 mice were injected i.p. with 200 mg/kg EC102 or equivalent vehicle control (Con) to demonstrate the latency in Hsp70 induction following Hsp90 inhibition. After 6 hours, a slight increase in Hsp70 levels was observed in EC102-treated brain tissue, followed by a robust induction at 24 hours compared with vehicle-treated brain tissue. (C) HeLa cells overexpressing V5-tau were treated with a 1-μM concentration of EC102 for the indicated time points. p-tau, Hsp70, and GAPDH levels were assessed by Western blot. p-tau levels were modestly decreased 6 hours after treatment, with maximal reduction seen at 24 hours after treatment. Hsp70 levels were increased in a time-dependent manner. (D) In-cell Western analysis showed that 60%–65% of total tau was converted to p-tau in HeLa cells 24 hours after transfection with V5-tau.
Figure 2. Proposed mechanism of chaperone/client pathway.
A substrate may initially be recognized by the Hsp40/Hsp70 complex with CHIP as the cochaperone/E3 ubiquitin (Ub) ligase. Transfer of the substrate to the Hsp90 complex is facilitated by Hop. There remain 2 fates for the substrate: dephosphorylation and refolding or ubiquitin-dependent proteasomal degradation. The mechanisms dictating which pathway is taken remain undefined. Hsp90 expression inhibits HSF1 activity by direct binding, which prevents HSF1 phosphorylation and trimerization. When Hsp90 is inhibited, Hsp90 levels are decreased, releasing HSF1, which in turn promotes de novo transcription of Hsps.
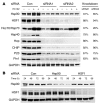
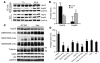
Figure 3. Targeted knockdown of primary components of the chaperone pathway promotes robust and rapid reductions in protein levels.
(A) HeLa cells were transfected in duplicate with a scrambled nonsilencing siRNA control or 2 independent siRNAs per gene, targeting the indicated components of the chaperone cycle. After 72 hours, the cells were treated with EC102 (1 μM) for an additional 24 hours and harvested for Western analyses. Knockdown efficiency at the protein level for both siRNAs targeting 7 of the 8 genes was greater than 60% and averaged 80%. Only siRNAs targeting Hsp70 had modest reductions of approximately 30%. Induction of Hsps by EC102 was also prevented by their knockdown, with the exception of Hsp70. GAPDH immunoreactivity was assessed on each membrane to control for loading differences. The panel shown is representative for all membranes. Based on similar knockdown efficiencies, these siRNAs were pooled for subsequent studies. Quantitation was assessed by densitometry. (B) HeLa cells were transfected with either nonsilencing siRNA control or 2 siRNA pools targeting Hsp90 or HSF1. The cells were harvested at the indicated time points. Partial knockdown was apparent after 24 hours for both genes, and immunoreactivity decreased further with each additional 24-hour interval. The 72-hour time point was chosen for subsequent studies to avoid issues associated with toxicity.
Figure 4. Reductions in p-tau by Hsp90 inhibition are primarily mediated by a constitutive, not an inducible, chaperone response.
(A) HeLa cells were transfected in duplicate with nonsilencing control, Hsp90, or HSF1 siRNA pools and incubated for 72 hours. The cells were then transfected with V5-tau and harvested after 24 hours’ EC102 exposure. EC102 caused robust reductions in p-tau and V5 immunoreactivity in cells transfected with a nonsilencing control or HSF1 siRNA (approximately 35%), while Hsp90 knockdown prevented this reduction. Densitometric values for Hsp90 and HSF1 siRNA pools are represented in separate graphs as a percentage of the optical density for nontransfected, vehicle-treated control cells after GAPDH normalization (dashed line). (B) HSF1 knockdown prevented Hsp40 and Hsp70 induction by EC102, while Hsp90 knockdown had no effect on either of these Hsps. Hsp90 levels were unaffected by HSF1 knockdown. EC102 treatment caused a shift in the distribution of HSF1 species, suggestive of phosphodependent activation.
Figure 5. Other components of the constitutive chaperone system are required to facilitate degradation of aberrant tau species by Hsp90 inhibitors; in turn, refolding machinery prevents p-tau degradation.
(A) HeLa cells were transfected in duplicate with nonsilencing control, CHIP, Hop, Hsp40, or Hsp70 siRNA pools for 72 hours, then transfected with V5-tau and harvested after 24 hours’ EC102 exposure. EC102 caused robust reductions in p-tau immunoreactivity in control-transfected cells, while chaperone knockdown prevented such reductions. (B) Densitometric values, shown as percent optical density of nontransfected, vehicle-treated (Veh) control cells after GAPDH normalization (dashed line). (C) P23 and Pin1 siRNA pools mimicked EC102 treatment. P23 knockdown reduced tau to an extent similar to that of EC102, whereas Pin1 knockdown reduced p-tau levels by nearly 75%. These siRNAs had no apparent effect on drug efficacy. (D) Effects of chaperone knockdown on total tau levels regardless of EC102 treatment were assessed by V5 immunoreactivity (including Hsp90 and HSF1 from Figure 4A). Densitometric values are shown as percent optical density of V5 immunoreactivity in nonsilencing siRNA–transfected cells after GAPDH normalization. Hsp90, CHIP, Hop, and Hsp40 all caused tau levels to accumulate by approximately 40%. HSF1 and Hsp70 had no effect on tau accumulation. P23 and Pin1 reduced total tau by approximately 35% and 75%, respectively. (E) These results suggest that dephosphorylation and refolding of p-tau is initially facilitated by an Hsp90/P23/Pin1 complex, preventing degradation; however, when refolding is subverted by Hsp90 inhibition or P23/Pin1 knockdown, p-tau is transferred to the Hsp70/CHIP complex, and polyubiquitination mediates degradation.
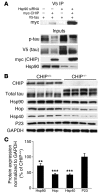
Figure 6. The unique cochaperone CHIP is essential for Hsp90 inhibitor–mediated tau degradation and regulates the levels of other chaperones as well as Hsp90.
(A) HeLa cells transfected with Hsp90 siRNA were subsequently transfected with V5-tau with or without myc-CHIP. Tau accumulated when Hsp90 expression was reduced; however, this accumulation was abrogated by CHIP and the amount of coimmunoprecipitated tau/CHIP complexes increased in the absence of Hsp90. (B) Chaperone protein levels were assessed in CHIP–/– brain tissue by Western blot analysis. The absence of CHIP and elevation in total tau levels were confirmed. Both Hsp40 and Hsp90 levels were decreased. In addition, the non–HSF1-mediated cochaperone, Hop, was also significantly decreased in CHIP–/– mice. P23 levels remained unchanged compared to GAPDH levels. (C) Quantification was assessed by standard densitometry. Error bars represent SD of the 4 CHIP–/– mice. **P < 0.01; ***P < 0.001 versus CHIP+/+.
Figure 7. Phosphorylation at S262/S356 prevents ubiquitination and degradation of tau by either CHIP or Hsp90 inhibition, but does not abolish CHIP/Hsp90 binding, further implicating ubiquitination of p-tau by CHIP as a necessary component for Hsp90-mediated degradation.
(A) HEK293 cells transfected with either wild-type V5-tau or V5-tau harboring the double mutation of S262A/S356A were cotransfected with myc-CHIP, PAR-1, or both. V5 coimmunoprecipitation showed that CHIP bound to and greatly enhanced the polyubiquitination of wild-type tau; however, PAR-1 phosphorylation of tau prevented this interaction. Mutation of the S262 and S356 sites to alanine residues attenuated CHIP binding; however, ubiquitination activity was entirely abrogated. Inputs confirmed the hyperphosphorylation of wild-type tau at the S262/S356 residues in the presence of PAR-1. (B) HeLa cells expressing wild-type V5-tau were cotransfected with constitutively active GSK3β, PAR-1, or empty vector and then treated with EC102 or vehicle. In the absence of EC102, constutively active GSK3β promoted the phosphorylation of tau at S396/S404 relative to empty vector and PAR-1 promoted the phosphorylation of tau at S262/S356 relative to empty vector. In the presence of EC102, phosphorylated S396/S404 was dramatically reduced compared with treatment with vehicle, while phosphorylated S262/S356 was largely unaffected by drug treatment. (C) HeLa cells expressing wild-type V5-tau were cotransfected with constitutively active GSK3β or empty vector. Tau was then coimmunoprecipitated with the V5 antibody, and Hsp90 binding was assessed. Hsp90 bound more avidly to tau phosphorylated by constutively active GSK3β compared with cells transfected with vector alone.
Figure 8. EC102 crosses the blood-brain barrier and acts as an Hsp90 inhibitor, decreasing p-tau levels in a mouse model of tauopathy.
(A and B) Mice (n = 7 per group) were injected i.p. with either 200 mg/kg EC102 or equivalent vehicle once daily for 7 days. Following the final injection, 5 mice were killed within 1 hour, the 2 remaining mice were harvested 24 hours later, and their brain homogenates were assessed by Western blot (A) and those of the 5 animals harvested after 1 hour were quantitated by densitometry normalized to GAPDH (B). Hsp70 levels showed a significant increase compared with vehicle-treated mice that persisted for 24 hours. Conversely, Hsp90 levels were significantly reduced only 1 hour after EC102 administration, but were indistinguishable from vehicle-treated animals within 24 hours. Values represent optical density ± SD. (C and D) Two cohorts of four 13- to 14-month-old Htau mice were injected i.p. with either EC102 or vehicle as above, all tissue was harvested 24 hours following the final injection, and Western blot (C) and densitometric quantitation for total tau (D) were performed. Dramatic reductions in phosphorylated S396/S404 and S202/T205 immunoreactivity in EC102-treated mice were observed. Hsp70 levels were significantly elevated in EC102-treated animals. Only the high–molecular weight species of tau (60–65 kDa), presumably p-tau species, were reduced by EC102 treatment; normal tau species (45–55 kDa) remained unaffected. Levels of Hsp90 clients Cdk5 and Akt were unaltered by Hsp90 inhibition. Error bars represent SD. *P < 0.05; **P < 0.001 versus vehicle.
Figure 9. Hsp90 expressed in affected tissue of AD brains has significantly increased binding affinity for EC102.
Brain homogenates from affected (temporal cortex, Cx) or unaffected (cerebellar cortex, Ceb) areas of 3 AD patients’ brains and homogenates from the same areas in brains of 3 control cases were evaluated for binding affinity to Hsp90 inhibitors in a competitive binding assay using a biotin-GA probe and increasing concentrations of EC102. Hsp90 derived from the temporal cortices of each AD patient showed 1,000-fold greater binding affinity for EC102, with an IC50 of 6 ± 3.6 nM, compared with Hsp90 from the cerebella, which had an IC50 of 6,000 ± 1,000 nM (P < 0.01). Controls had an IC50 of 6,000 ± 2,000 nM and 7,333 ± 2,081 nM in the temporal and cerebellar cortices, respectively. An example of the Hsp90 levels from a case and control from each area examined following competition assay between biotin-GA and EC102. Note the similar levels of Hsp90 present at 0 μM EC102 among all tissues examined.
Comment in
- CHIP-ping away at tau.
Goryunov D, Liem RK. Goryunov D, et al. J Clin Invest. 2007 Mar;117(3):590-2. doi: 10.1172/JCI31505. J Clin Invest. 2007. PMID: 17332887 Free PMC article.
Similar articles
- Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer's disease.
Salminen A, Ojala J, Kaarniranta K, Hiltunen M, Soininen H. Salminen A, et al. Prog Neurobiol. 2011 Jan;93(1):99-110. doi: 10.1016/j.pneurobio.2010.10.006. Epub 2010 Nov 5. Prog Neurobiol. 2011. PMID: 21056617 Review. - CHIP-ping away at tau.
Goryunov D, Liem RK. Goryunov D, et al. J Clin Invest. 2007 Mar;117(3):590-2. doi: 10.1172/JCI31505. J Clin Invest. 2007. PMID: 17332887 Free PMC article. - Tau phosphorylation, molecular chaperones, and ubiquitin E3 ligase: clinical relevance in Alzheimer's disease.
Kumar P, Jha NK, Jha SK, Ramani K, Ambasta RK. Kumar P, et al. J Alzheimers Dis. 2015;43(2):341-61. doi: 10.3233/JAD-140933. J Alzheimers Dis. 2015. PMID: 25096626 Review. - In vivo evidence of CHIP up-regulation attenuating tau aggregation.
Sahara N, Murayama M, Mizoroki T, Urushitani M, Imai Y, Takahashi R, Murata S, Tanaka K, Takashima A. Sahara N, et al. J Neurochem. 2005 Sep;94(5):1254-63. doi: 10.1111/j.1471-4159.2005.03272.x. J Neurochem. 2005. PMID: 16111477 - Hsp90 activator Aha1 drives production of pathological tau aggregates.
Shelton LB, Baker JD, Zheng D, Sullivan LE, Solanki PK, Webster JM, Sun Z, Sabbagh JJ, Nordhues BA, Koren J 3rd, Ghosh S, Blagg BSJ, Blair LJ, Dickey CA. Shelton LB, et al. Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):9707-9712. doi: 10.1073/pnas.1707039114. Epub 2017 Aug 21. Proc Natl Acad Sci U S A. 2017. PMID: 28827321 Free PMC article.
Cited by
- Co-Chaperones in Targeting and Delivery of Misfolded Proteins to the 26S Proteasome.
Abildgaard AB, Gersing SK, Larsen-Ledet S, Nielsen SV, Stein A, Lindorff-Larsen K, Hartmann-Petersen R. Abildgaard AB, et al. Biomolecules. 2020 Aug 4;10(8):1141. doi: 10.3390/biom10081141. Biomolecules. 2020. PMID: 32759676 Free PMC article. Review. - CHIP as a therapeutic target for neurological diseases.
Zhang S, Hu ZW, Mao CY, Shi CH, Xu YM. Zhang S, et al. Cell Death Dis. 2020 Sep 9;11(9):727. doi: 10.1038/s41419-020-02953-5. Cell Death Dis. 2020. PMID: 32908122 Free PMC article. Review. - Promoting Neuronal Tolerance of Diabetic Stress: Modulating Molecular Chaperones.
Emery SM, Dobrowsky RT. Emery SM, et al. Int Rev Neurobiol. 2016;127:181-210. doi: 10.1016/bs.irn.2016.03.001. Epub 2016 Apr 5. Int Rev Neurobiol. 2016. PMID: 27133150 Free PMC article. Review. - Lysosomal fusion dysfunction as a unifying hypothesis for Alzheimer's disease pathology.
Funk KE, Kuret J. Funk KE, et al. Int J Alzheimers Dis. 2012;2012:752894. doi: 10.1155/2012/752894. Epub 2012 Aug 30. Int J Alzheimers Dis. 2012. PMID: 22970406 Free PMC article. - Loss of HDAC6, a novel CHIP substrate, alleviates abnormal tau accumulation.
Cook C, Gendron TF, Scheffel K, Carlomagno Y, Dunmore J, DeTure M, Petrucelli L. Cook C, et al. Hum Mol Genet. 2012 Jul 1;21(13):2936-45. doi: 10.1093/hmg/dds125. Epub 2012 Apr 5. Hum Mol Genet. 2012. PMID: 22492994 Free PMC article.
References
- Hutton M., et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. - PubMed
- Feany M.B., Dickson D.W. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann. Neurol. 1996;40:139–148. - PubMed
- Gomez-Isla T., et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann. Neurol. 1997;41:17–24. - PubMed
- Braak H., Braak E. Neuropathological staging of Alzheimer-related changes. Acta. Neuropathol. (Berl.). 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases