NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus - PubMed (original) (raw)
doi: 10.1105/tpc.106.046938. Epub 2007 Feb 16.
Makoto Yoshikawa, Koji Yano, Hiroki Miwa, Hisaki Uchida, Erika Asamizu, Shusei Sato, Satoshi Tabata, Haruko Imaizumi-Anraku, Yosuke Umehara, Hiroshi Kouchi, Yoshikatsu Murooka, Krzysztof Szczyglowski, J Allan Downie, Martin Parniske, Makoto Hayashi, Masayoshi Kawaguchi
Affiliations
- PMID: 17307929
- PMCID: PMC1867344
- DOI: 10.1105/tpc.106.046938
NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus
Katsuharu Saito et al. Plant Cell. 2007 Feb.
Abstract
In Lotus japonicus, seven genetic loci have been identified thus far as components of a common symbiosis (Sym) pathway shared by rhizobia and arbuscular mycorrhizal fungi. We characterized the nup85 mutants (nup85-1, -2, and -3) required for both symbioses and cloned the corresponding gene. When inoculated with Glomus intraradices, the hyphae managed to enter between epidermal cells, but they were unable to penetrate the cortical cell layer. The nup85-2 mutation conferred a weak and temperature-sensitive symbiotic phenotype, which resulted in low arbuscule formation at 22 degrees C but allowed significantly higher arbuscule formation in plant cortical cells at 18 degrees C. On the other hand, the nup85 mutants either did not form nodules or formed few nodules. When treated with Nod factor of Mesorhizobium loti, nup85 roots showed a high degree of root hair branching but failed to induce calcium spiking. In seedlings grown under uninoculated conditions supplied with nitrate, nup85 did not arrest plant growth but significantly reduced seed production. NUP85 encodes a putative nucleoporin with extensive similarity to vertebrate NUP85. Together with symbiotic nucleoporin NUP133, L. japonicus NUP85 might be part of a specific nuclear pore subcomplex that is crucial for fungal and rhizobial colonization and seed production.
Figures
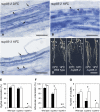
Figure 1.
Root Hair Deformation, Infection Thread Formation, and Calcium Spiking. Root hair responses after treatment with 10−8 M Nod factor for 24 h. (A) Wild-type control. (B) Wild type with Nod factor treatment. (C) nup85-2 control. (D) nup85-2 Nod factor treatment. (E) nup85-3 control. (F) nup85-3 Nod factor treatment. The photos are representative of observations of at least 15 plants in each line. Bar = 100 μm. (G) Number of infection thread of the wild type, nup85-2, and nup85-3. The data presented are means ±
se
(n = 6). Different letters above bars indicate significance among the means according to the Tukey multiple comparison test (P < 0.05). Analysis of calcium spiking in root hair. (H) Each trace is from a single root hair using seedlings of the wild type, nup85-2, or nup85-3 mutant. Root hairs were injected with the calcium-sensitive dye Oregon green dextran, and after ∼20 min, Nod factor was added at 10−8 M. The data are graphed showing typical traces of the differences in fluorescence intensity between 5-s sequential time points. Only viable cells showing active cytoplasmic streaming were used in the analysis. These cells were observed for at least 60 min following Nod factor treatments. The fractions in parentheses show the numbers of root hairs in which calcium spiking was detected to the number of root hairs tested.
Figure 2.
AM Colonization. (A) Wild-type plant inoculated with G. intraradices. G. intraradices formed appressoria (Ap) and entered between epidermal (Ep) cells. The hyphae penetrated the cortical (C) cells. The arrowhead shows the entry point of the hyphae into plant cells. The hyphae spread into the intercellular space of the cortex and then penetrated into inner cortical cells, where arbuscules (Ar) formed. (B) nup85-2 mutant inoculated with G. intraradices. The hyphae from appressoria could enter the epidermis but could not penetrate the outer cortical cells. The hypha (asterisk) has swollen at the epidermis/cortex interface. (C) and (D) Different focal planes of the root surface of the nup85-3 mutant inoculated with G. intraradices: surface of epidermal cells (C) and cell layer of outer cortex (D). Fungus has formed an appressorium on the root surface and can enter between epidermal cells (arrowheads). However, the hypha has stopped its growth and swollen at the epidermis/cortex interface. The asterisk indicates the swelling structure. The photos are representative of observations of at least six plants from each plant line. Bars = 50 μm.
Figure 3.
Temperature Effects on Mycorrhization and Nodulation. (A) The nup85-2 mutant inoculated with G. intraradices and grown at 22°C. Hyphae (arrows) spread into an intercellular space of the cortex; however, few arbuscules (arrowhead) were formed in cortical cells. Bar = 100 μm. (B) Mycorrhizal roots of the nup85-2 mutant grown at 18°C. A hypha spread into the intercellular space of the cortex and penetrated into inner cortical cells, where arbuscules (arrowheads) formed as infected in the wild type. Bar = 100 μm. (C) nup85-3 mutant inoculated with G. intraradices and grown at 18°C. The hypha entered epidermis and formed swollen structures (arrow) at the epidermis/cortex interface. The hyphae emerging from the swollen structures penetrated to the outer cortical cells and spread into intercellular space of cortex. Arbuscules (arrowheads) formed in inner cortical cells. Bar = 100 μm. (D) Nodule formation of the wild type, nup85-2, and nup85-3 mutants grown at 22°C and 18°C. The number of nodule varied at different temperatures. Arrowheads indicate visible nodules. Bar = 1 cm. (E) to (G) Hyphal colonization (E), arbuscular colonization (F), and number of nodules (G) of the wild type, nup85-2, and nup85-3 mutants grown at 22°C (closed bars) and 18°C (open bars). Error bars indicate SE (n = 3). For assessing rates of mycorrhizal colonization, at least 100 points in the mycorrhizal roots were analyzed in each replicate. Asterisks show significant differences between 22°C and 18°C by t test: * P < 0.05 and ** P < 0.01.
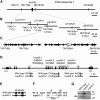
Figure 4.
Positional Cloning of the NUP85 Gene. (A) Genetic map of the NUP85 gene on chromosome 1. Name and positions of the molecular markers are indicated. (B) Physical map of the TAC/BAC clones. The names of the clones are indicated above the lines. The number of recombination events that occurred between the NUP85 locus and the molecular markers are indicated below the lines. Note that there is a gap between LjB18I12 and LjT40A22. (C) Predicted open reading frames between molecular markers TM1792b and BM1918b. The region contains 37 open reading frames (arrows). The NUP85 gene is indicated as a white arrow. (D) Representation of the exon and intron organization of the NUP85 gene. The gene consists of 18 exons (boxes) and 17 introns. The positions of the mutations in the nup85-1, -2, -3, and -4 alleles are indicated the line above. Roman numerals above the boxes indicate the exon order. nup85-1 has a nonsense mutation in exon VIII. The partial genomic sequence of the wild type, nup85-2, and nup85-3 are shown. Uppercase and lowercase characters indicate sequence in exons and introns, respectively. The positions of mutations are shaded in gray. nup85-2, -3, and -4 have a point mutation in the 13th, 11th, and eighth introns, respectively. (E) Sequence analysis of NUP85 cDNA in the nup85-2 mutant. Boxes indicate transcribed sequences on the genomic sequence. The mutation in the nup85-2 mutant results in a deletion of exon XIII in the transcript. (F) Sequence comparison between NUP85 cDNAs of the wild type and nup85-3 mutant. Roman numerals indicate the exon order. The transcript of the nup85-3 mutant has a four-base deletion, which results in a premature stop codon. (G) The wild type, nup85-2, and nup85-3 were subjected to immunoblot analyses with anti-NUP85 antibodies. Molecular masses are given at the right in kilodaltons. Arrow indicates predicted molecular mass of NUP85.
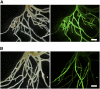
Figure 5.
Complementation of the nup85-3 Mutant with the Putative Nucleoporin-Like Gene in TAC Clone LjT40A22. (A) A full-length cDNA of the putative nucleoporin-like gene was introduced into nup85-3 using _A. rhizogenes_–mediated hairy root transformation. GFP fluorescence indicates transformed hairy roots. Transformed roots developed nodules after inoculation with M. loti. (B) Roots of nup85-3 were transformed with the empty vector pGUN. The transformed plants do not form nodules. Bars = 2 mm.
Figure 6.
Amino Acid Sequence Alignment and DNA Hybridization Analysis of NUP85. (A) Amino acid sequence alignment of L. japonicus NUP85, _Arabidopsis NUP85_-like gene (At4g32910), and human nucleoporin 75 (huNup75). Identical and similar amino acids are shaded in black and gray, respectively. DNA hybridization analysis of L. japonicus Gifu genomic DNA hybridized with NUP85 probes. (B) The DNA was digested with _Eco_RI or _Hin_dIII. NUP85 probes A and B recognize the 5′ and 3′ regions of the NUP85 gene, respectively. Arrows indicate extra fragments that were not predicted from the genomic sequence of the NUP85 gene. (C) Genomic sequences of the NUP85 gene and a partial gene (BM1990.3) on clone LjB03D18. The region indicated by the arrow is highly conserved between the genes. Regions of genomic sequence hybridized with probes A and B were indicated. Filled boxes indicate predicted exons. Closed and open triangles show _Eco_RI and _Hin_dIII sites, respectively.
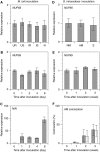
Figure 7.
Gene Expression. (A) to (C) M. loti inoculation. (A) Expression of NUP85 in uninfected roots (UR), shoots of uninoculated plants (US), infected roots (IR), shoots of inoculated plants (IS), and nodules (N) at 3 weeks after inoculation treatment. Expression levels are normalized on the basis of the amount of EF-1 and expressed relative to uninfected roots. (B) and (C) Expression of NUP85 and NIN from 0 to 8 d following inoculation. Expression levels are expressed relative to day 0. (D) to (F) G. intraradices inoculation. (D) Expression of NUP85 in nonmycorrhizal roots (NM), mycorrhizal roots (AM), and shoots of mycorrhizal plants (S) at 3 weeks after inoculation treatment. Expression levels are normalized on the basis of the amount of EF-1 and expressed relative to nonmycorrhizal roots. (E) Expression of NUP85 from 0 to 3 weeks following inoculation. Expression levels are expressed relative to day 0. (F) AM colonization. Gray and white bars show hyphal colonization (HC%) and arbuscular colonization (AC%), respectively. Values are the means of three experiments of different samples.
sd
is indicated.
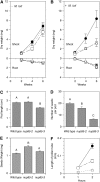
Figure 8.
Plant Growth and Seed Production. (A) Dry weight of shoots and roots in the wild type (closed circles), nup85-2 (open squares), and nup85-3 (open triangles) mutants supplied with BandD solution containing 5 mM KNO3, without M. loti inoculation. (B) Dry weight of the plants inoculated with M. loti and supplied with nitrogen-free BandD solution. Error bars show
se
of the means (n = 3). (C) Pod length of the wild type, nup85-2, and nup85-3 grown in a complete nutrient-rich medium. (D) Number of mature seeds per pod. (E) Seed weight. The data presented are means ±
se
(n = 37 to 40). Different letters above bars indicate significance among the means according to the Tukey multiple comparison test (P < 0.05). (F) Length of pollen tubes in the wild type (closed circles), nup85-2 (open squares), and nup85-3 (open triangles) at 1 and 2 h after pollen germination. At least 13 pollen tubes were observed. Error bars show
se
.
Similar articles
- Two Lotus japonicus symbiosis mutants impaired at distinct steps of arbuscule development.
Groth M, Kosuta S, Gutjahr C, Haage K, Hardel SL, Schaub M, Brachmann A, Sato S, Tabata S, Findlay K, Wang TL, Parniske M. Groth M, et al. Plant J. 2013 Jul;75(1):117-129. doi: 10.1111/tpj.12220. Epub 2013 Jun 10. Plant J. 2013. PMID: 23627596 - Rhizobial infection does not require cortical expression of upstream common symbiosis genes responsible for the induction of Ca(2+) spiking.
Hayashi T, Shimoda Y, Sato S, Tabata S, Imaizumi-Anraku H, Hayashi M. Hayashi T, et al. Plant J. 2014 Jan;77(1):146-59. doi: 10.1111/tpj.12374. Epub 2013 Dec 12. Plant J. 2014. PMID: 24329948 Free PMC article. - A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis.
Kanamori N, Madsen LH, Radutoiu S, Frantescu M, Quistgaard EM, Miwa H, Downie JA, James EK, Felle HH, Haaning LL, Jensen TH, Sato S, Nakamura Y, Tabata S, Sandal N, Stougaard J. Kanamori N, et al. Proc Natl Acad Sci U S A. 2006 Jan 10;103(2):359-64. doi: 10.1073/pnas.0508883103. Epub 2006 Jan 3. Proc Natl Acad Sci U S A. 2006. PMID: 16407163 Free PMC article. - Spontaneous symbiotic reprogramming of plant roots triggered by receptor-like kinases.
Ried MK, Antolín-Llovera M, Parniske M. Ried MK, et al. Elife. 2014 Nov 25;3:e03891. doi: 10.7554/eLife.03891. Elife. 2014. PMID: 25422918 Free PMC article. - The Lotus japonicus LjSym4 gene is required for the successful symbiotic infection of root epidermal cells.
Bonfante P, Genre A, Faccio A, Martini I, Schauser L, Stougaard J, Webb J, Parniske M. Bonfante P, et al. Mol Plant Microbe Interact. 2000 Oct;13(10):1109-20. doi: 10.1094/MPMI.2000.13.10.1109. Mol Plant Microbe Interact. 2000. PMID: 11043472
Cited by
- How membranes shape plant symbioses: signaling and transport in nodulation and arbuscular mycorrhiza.
Bapaume L, Reinhardt D. Bapaume L, et al. Front Plant Sci. 2012 Oct 5;3:223. doi: 10.3389/fpls.2012.00223. eCollection 2012. Front Plant Sci. 2012. PMID: 23060892 Free PMC article. - Signaling and Detoxification Strategies in Plant-Microbes Symbiosis under Heavy Metal Stress: A Mechanistic Understanding.
Liu Y, He G, He T, Saleem M. Liu Y, et al. Microorganisms. 2022 Dec 26;11(1):69. doi: 10.3390/microorganisms11010069. Microorganisms. 2022. PMID: 36677361 Free PMC article. Review. - Identification and characterization of the Arabidopsis FG-repeat nucleoporin Nup62.
Zhao Q, Meier I. Zhao Q, et al. Plant Signal Behav. 2011 Mar;6(3):330-4. doi: 10.4161/psb.6.3.13402. Plant Signal Behav. 2011. PMID: 21673506 Free PMC article. - Functional adaptation of a plant receptor-kinase paved the way for the evolution of intracellular root symbioses with bacteria.
Markmann K, Giczey G, Parniske M. Markmann K, et al. PLoS Biol. 2008 Mar 4;6(3):e68. doi: 10.1371/journal.pbio.0060068. PLoS Biol. 2008. PMID: 18318603 Free PMC article. - Calcium spiking patterns and the role of the calcium/calmodulin-dependent kinase CCaMK in lateral root base nodulation of Sesbania rostrata.
Capoen W, Den Herder J, Sun J, Verplancke C, De Keyser A, De Rycke R, Goormachtig S, Oldroyd G, Holsters M. Capoen W, et al. Plant Cell. 2009 May;21(5):1526-40. doi: 10.1105/tpc.109.066233. Epub 2009 May 26. Plant Cell. 2009. PMID: 19470588 Free PMC article.
References
- Ané, J.-M., et al. (2004). Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303 1364–1367. - PubMed
- Asamizu, E., Nakamura, Y., Sato, S., and Tabata, S. (2004). Characteristics of the Lotus japonicus gene repertoire deduced from large-scale expressed sequence tag (EST) analysis. Plant Mol. Biol. 54 405–414. - PubMed
- Bonfante, P., Genre, A., Faccio, A., Martini, I., Schauser, L., Stougaard, J., Webb, J., and Parniske, M. (2000). The Lotus japonicus LjSym4 gene is required for the successful symbiotic infection of root epidermal cells. Mol. Plant Microbe Interact. 13 1109–1120. - PubMed
- Cárdenas, L., Feijó, J.A., Kunkel, J.G., Sánchez, F., Holdaway-Clarke, T., Hepler, P.K., and Quinto, C. (1999). Rhizobium Nod factors induce increases in intracellular free calcium and extracellular calcium influxes in bean root hairs. Plant J. 19 347–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical