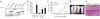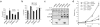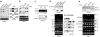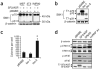The gene encoding the splicing factor SF2/ASF is a proto-oncogene - PubMed (original) (raw)
The gene encoding the splicing factor SF2/ASF is a proto-oncogene
Rotem Karni et al. Nat Struct Mol Biol. 2007 Mar.
Abstract
Alternative splicing modulates the expression of many oncogene and tumor-suppressor isoforms. We have tested whether some alternative splicing factors are involved in cancer. We found that the splicing factor SF2/ASF is upregulated in various human tumors, in part due to amplification of its gene, SFRS1. Moreover, slight overexpression of SF2/ASF is sufficient to transform immortal rodent fibroblasts, which form sarcomas in nude mice. We further show that SF2/ASF controls alternative splicing of the tumor suppressor BIN1 and the kinases MNK2 and S6K1. The resulting BIN1 isoforms lack tumor-suppressor activity; an isoform of MNK2 promotes MAP kinase-independent eIF4E phosphorylation; and an unusual oncogenic isoform of S6K1 recapitulates the transforming activity of SF2/ASF. Knockdown of either SF2/ASF or isoform-2 of S6K1 is sufficient to reverse transformation caused by the overexpression of SF2/ASF in vitro and in vivo. Thus, SF2/ASF can act as an oncoprotein and is a potential target for cancer therapy.
Figures
Figure 1
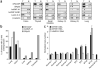
Upregulation and amplification of SF2/ASF in human tumors. (a) A protein microarray (Biochain) consisting of 47 pairs of tumor samples (T) and their normal tissue counterparts (N), spotted as duplicates, was probed with the indicated antibodies. Representative tumor samples overexpressing SF2/ASF and corresponding controls: colon, adenocarcinoma; thyroid, follicular carcinoma; small intestine, leiomyoma; kidney (1), clear cell carcinoma; kidney (2), granular cell carcinoma. (b) Expression of splicing factor mRNA was measured by reverse-transcription quantitative PCR (RT-qPCR) in 50 tumor samples and five normal samples from each indicated tissue and normalized to β-actin mRNA. Bars show the proportion of tumors overexpressing the mRNA more than two-fold over the mean of the five normal samples. Expression of hnRNP A1 was determined only in lung and colon tumors. (c) DNA and RNA were measured by qPCR and RT-qPCR in 50 breast tumor samples and five normal samples. Data for the four tumors (infiltrating ductal carcinomas) with elevated DNA copy numbers of SF2/ASF are shown relative to the mean of the normal samples. Normal BT10, surrounding normal breast tissue from the patient with the BT10 tumor. Means ± s.d. from triplicate qPCR measurements are shown. *P = 4.9 times; 10−10 for the BT10 versus normal BT10 comparison; n = 6.
Figure 2
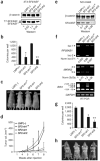
SF2/ASF transforms immortal cells and is tumorigenic in nude mice. (a) Total proteins from duplicate pools of NIH 3T3 stable cell lines transduced with retroviruses expressing LacZ or T7-tagged SF2/ASF, SRp55, SC35 or hnRNP A1 were analyzed by western blotting with anti-T7. The first four samples were also analyzed with anti-SF2/ASF (below) to compare the expression of endogenous and transduced SF2/ASF (b) Quantification of soft-agar colony formation by the stably transduced cell lines. The mean ± s.d. for each pair of pooled lines is shown. _P_-values in pairwise comparisons to the LacZ control: *_P_=4 × 10−8; **P = 10−7; ***P = 7 × 10−8. (c) Tumor growth curve in mice injected with 2 × 106 cells from the indicated NIH 3T3 pooled lines. The number of tumors formed per number of injections is shown in parentheses. SRp55 and SC35 cell lines (six each) did not form tumors during the same time course (data not shown). Error bars, s.d. (d) Representative mice injected with LacZ-expressing control cells, or with SF2/ASF-overexpressing cells. (e) Light micrographs of formalin-fixed, paraffin-embedded tissue sections from tumors derived from NIH 3T3 cells overexpressing SF2/ASF of hnRNP A1, stained with hematoxylin and eosin. Scale bars, 100 μm.
Figure 3
SF2/ASF overexpression protects E1A-sensitized MEF cells against apoptosis and enhances the proliferation of Ras-transformed cells. Primary wild-type MEF cells were transduced with retroviruses expressing the indicated human splicing factor cDNAs, or with the empty vector; some cells were cotransduced with adenovirus E1A or activated Ras, as indicated. Error bars indicate s.d. (a) After drug selection, cells coinfected with E1A were plated and serum-starved, and cell death was measured by trypan blue staining. _P_-values in pairwise comparisons to the pBABE control: *P = 5× 10−8; **P = 5 × 10−5; n = 3. (b) Cells coinfected with E1A were plated and treated with the indicated adriamycin concentrations after 24 h, and cell death was measured as in a. *P = 0.003, **P = 0.01, ***P = 0.006; n = 3; for SF2/ASF at low adriamycin, n = 1. (c) MEF cells were infected with the indicated retroviruses, plated after single or double selection (106 cells per 10-cm plate) and lysed in SDS after 24 h. Western blots were carried out using the indicated primary antibodies. The reduction in SRp55 in the presence of E1A was not observed with other cell lines (data not shown). (d) p53−/− MEF cells were transduced with the empty vector or with retroviruses expressing SF2/ASF or SRp55, either alone or with oncogenic Ras. Cells were fixed at 48-h intervals and stained with methylene blue. Each point represents the mean relative absorbance from six wells.
Figure 4
Specific alternative splicing changes induced by overexpression of splicing factors or knockdown of SF2/ASF. (a) Human primary cells (IMR90) were infected with the indicated retroviruses, and stable pools were selected. Total RNA was analyzed by radioactive RT-PCR, nondenaturing PAGE and autoradiography using the indicated primers (arrowheads). GAPDH mRNA was analyzed as a control. The exon structure of each BIN1 isoform is indicated. (b) HeLa cells were transfected in duplicate with siRNAs specific for SF2/ASF or PP2Cγ, or mock-transfected. After 72 h, total RNA was analyzed as in a. (c) NIH 3T3 cell lines with inducible expression of SF2/ASF were plated and induced 24 h later with 5 μM ponasterone A for the indicated times. RNA was analyzed by radioactive RT-PCR; quantification of the proportion of exon inclusion (% incl) by phosphorimaging analysis is shown below the ethidium bromide–stained agarose gel. Only two isoforms were detected in these mouse cells. Expressed T7-tagged SF2/ASF and endogenous β-catenin were analyzed by western blotting. (d) Total RNA and protein were extracted from IMR90 primary cells overexpressing the indicated splicing factors. For both panels, total RNA was analyzed by RT-PCR using the indicated isoform-specific primers (arrowheads). Alternatively spliced exons are shaded. Western blotting was carried out as in c. (e) Total RNA and protein were extracted from duplicate samples of HeLa cells treated with siRNAs targeting SF2/ASF or PP2Cγ, or mock-treated. PP2Cγ and SF2/ASF protein levels were analyzed by western blotting. RNA samples were analyzed as in d.
Figure 5
SF2/ASF promotes expression of an oncogenic isoform of S6K1 and induces eIF4E phosphorylation. (a) MEF, NIH 3T3 and IMR90 cells were transduced with pBABE or SF2/ASF retroviruses and lysed in SDS. Western blotting was carried out using a monoclonal antibody against the N terminus of S6K1 to detect the endogenous isoforms and one against p-catenin as a loading control. (b) NIH 3T3 cells were transduced with pBABE or S6K1 isoform-1 or isoform-2 retroviruses, and lysates were analyzed by western blotting as in a. The S6K1 antibody reacts with both the endogenous and T7-tagged S6K1 isoforms, but the endogenous isoform-2 is not detected in this exposure. (c) Aliquots of cells in b were seeded in soft agar, and colonies were counted 14 d later. Means ± s.d. are shown; *P = 6×10−7 compared to the pBABE control. (d) MEF cells were transduced with the indicated retroviruses and analyzed by western blotting with the indicated antibodies.
Figure 6
Knockdown of SF2/ASF reverses transformation of NCI-H460 cells and SF2/ASF-overexpressing NIH 3T3 cells. (a) Western blot analysis of SF2/ASF in NIH 3T3 cells overexpressing SF2/ASF after transduction with viruses expressing the indicated SF2/ASF shRNAs. The SF2/ASF-specific shRNAs target the 3′ UTR of SF2/ASF and therefore affect only the endogenous SF2/ASF (lower band), for which normalized protein levels are shown under the blot. SF2-sh1m is a control shRNA with two mismatches. (b) Cells described in a were seeded in soft agar, and colonies were counted 14 d later; means ± s.d. are shown. _P_-values for comparisons to the LMP(–) control: *P = 2 × 10−7; **P = 2 × 10−9. (c,d) Cell lines described in a were injected into nude mice (n = 8 injections) and tumor volume was measured weekly; error bars indicate s.d. Representative mice are shown. (e) Western blot of total protein from NCI-H460 cells as in a. (f) RNA from the cells in e was analyzed by RT-PCR and RT-qPCR to detect MKNK2, RPS6KB1 and BIN1 isoform levels, with GAPDH as a control. Normalized ratios of RPS6KB1 isoform-2 to isoform-1 and MKNK2 2b to 2a calculated from the RT-qPCR data are shown under the corresponding gels. (g) Soft agar colony formation by cells described in e. Means ± s.d. are shown. P-values calculated as in b: *P = 3 × 10−11; **P = 2 × 10−15. (h) Cell lines described in a were injected into nude mice (n = 8 injections). Representative mice are shown.
Figure 7
Knockdown of S6K1 isoform-2 blocks SF2/ASF-mediated transformation. (a) Immunoprecipitation (IP)-western blot analysis of S6K1 isoforms in NIH 3T3 cells overexpressing SF2/ASF, after transduction with retroviruses expressing the indicated S6K1 isoform-specific shRNAs. (b) Cells described in a were seeded in soft agar and colonies were counted as in Figure 6b; means ± s.d. are shown. *P = 6×10−8.
Figure 8
A model for transformation by SF2/ASF. SF2/ASF (which can be blocked by specific shRNAs) activates several key targets that contribute to the transformed phenotype: induction of MNK2b promotes phosphorylation of eIF4E, which enhances cap-dependent translation; induction of the oncogenic S6K1 isoform-2 directly leads to transformation; and induction of inactive isoforms of the putative tumor suppressor BIN1 inhibits apoptosis. Other SF2/ASF targets are also likely to contribute to transformation.
Comment in
- Splicing oncogenes.
Hu A, Fu XD. Hu A, et al. Nat Struct Mol Biol. 2007 Mar;14(3):174-5. doi: 10.1038/nsmb0307-174. Nat Struct Mol Biol. 2007. PMID: 17334403 No abstract available.
Similar articles
- The splicing-factor oncoprotein SF2/ASF activates mTORC1.
Karni R, Hippo Y, Lowe SW, Krainer AR. Karni R, et al. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15323-7. doi: 10.1073/pnas.0801376105. Epub 2008 Oct 1. Proc Natl Acad Sci U S A. 2008. PMID: 18832178 Free PMC article. - Alternative splicing factor or splicing factor-2 plays a key role in intron retention of the endoglin gene during endothelial senescence.
Blanco FJ, Bernabeu C. Blanco FJ, et al. Aging Cell. 2011 Oct;10(5):896-907. doi: 10.1111/j.1474-9726.2011.00727.x. Epub 2011 Jul 19. Aging Cell. 2011. PMID: 21668763 - SF2/ASF autoregulation involves multiple layers of post-transcriptional and translational control.
Sun S, Zhang Z, Sinha R, Karni R, Krainer AR. Sun S, et al. Nat Struct Mol Biol. 2010 Mar;17(3):306-12. doi: 10.1038/nsmb.1750. Epub 2010 Feb 7. Nat Struct Mol Biol. 2010. PMID: 20139984 Free PMC article. - ASF/SF2: a splice site selector.
Lamond AI. Lamond AI. Trends Biochem Sci. 1991 Dec;16(12):452-3. doi: 10.1016/0968-0004(91)90176-v. Trends Biochem Sci. 1991. PMID: 1781021 Review. No abstract available. - Splicing Regulators and Their Roles in Cancer Biology and Therapy.
da Silva MR, Moreira GA, Gonçalves da Silva RA, de Almeida Alves Barbosa É, Pais Siqueira R, Teixera RR, Almeida MR, Silva Júnior A, Fietto JL, Bressan GC. da Silva MR, et al. Biomed Res Int. 2015;2015:150514. doi: 10.1155/2015/150514. Epub 2015 Jul 26. Biomed Res Int. 2015. PMID: 26273588 Free PMC article. Review.
Cited by
- The role of VEGF 165b in pathophysiology.
Peiris-Pagès M. Peiris-Pagès M. Cell Adh Migr. 2012 Nov-Dec;6(6):561-8. doi: 10.4161/cam.22439. Epub 2012 Oct 17. Cell Adh Migr. 2012. PMID: 23076130 Free PMC article. Review. - EVI1 splice variants modulate functional responses in ovarian cancer cells.
Dutta P, Bui T, Bauckman KA, Keyomarsi K, Mills GB, Nanjundan M. Dutta P, et al. Mol Oncol. 2013 Jun;7(3):647-68. doi: 10.1016/j.molonc.2013.02.008. Epub 2013 Mar 5. Mol Oncol. 2013. PMID: 23517670 Free PMC article. - Splicing factor SRSF3 is crucial for hepatocyte differentiation and metabolic function.
Sen S, Jumaa H, Webster NJ. Sen S, et al. Nat Commun. 2013;4:1336. doi: 10.1038/ncomms2342. Nat Commun. 2013. PMID: 23299886 Free PMC article. - Serine/arginine-rich splicing factors: the bridge linking alternative splicing and cancer.
Zheng X, Peng Q, Wang L, Zhang X, Huang L, Wang J, Qin Z. Zheng X, et al. Int J Biol Sci. 2020 Jul 6;16(13):2442-2453. doi: 10.7150/ijbs.46751. eCollection 2020. Int J Biol Sci. 2020. PMID: 32760211 Free PMC article. Review. - Splicing-factor alterations in cancers.
Anczuków O, Krainer AR. Anczuków O, et al. RNA. 2016 Sep;22(9):1285-301. doi: 10.1261/rna.057919.116. RNA. 2016. PMID: 27530828 Free PMC article. Review.
References
- Johnson JM, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. - PubMed
- Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. - PubMed
- Mayeda A, Krainer AR. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992;68:365–375. - PubMed
- Cáceres JF, Stamm S, Helfman DM, Krainer AR. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–1709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources