Natural mutations in the receptor binding domain of spike glycoprotein determine the reactivity of cross-neutralization between palm civet coronavirus and severe acute respiratory syndrome coronavirus - PubMed (original) (raw)
Comparative Study
. 2007 May;81(9):4694-700.
doi: 10.1128/JVI.02389-06. Epub 2007 Feb 21.
Qing Fang, Fei Deng, Hanzhong Wang, Christopher E Yi, Lei Ba, Wenjie Yu, Richard D Lin, Taisheng Li, Zhihong Hu, David D Ho, Linqi Zhang, Zhiwei Chen
Affiliations
- PMID: 17314167
- PMCID: PMC1900161
- DOI: 10.1128/JVI.02389-06
Comparative Study
Natural mutations in the receptor binding domain of spike glycoprotein determine the reactivity of cross-neutralization between palm civet coronavirus and severe acute respiratory syndrome coronavirus
Li Liu et al. J Virol. 2007 May.
Abstract
The severe acute respiratory syndrome (SARS) outbreak of 2002 and 2003 occurred as a result of zoonotic transmission. Coronavirus (CoV) found in naturally infected palm civet (civet-CoV) represents the closest genetic relative to SARS-CoV, but the degree and the determinants of cross-neutralization among these viruses remain to be investigated. Studies indicate that the receptor binding domain (RBD) of the SARS-CoV spike (S) glycoprotein contains major determinants for viral entry and neutralization. We aim to characterize the impact of natural mutations within the RBDs of civet-CoVs on viral entry and cross-neutralization. In this study, the S glycoprotein genes were recovered from naturally infected civets in central China (Hubei province), extending the geographic distribution of civet-CoV beyond the southeastern province of Guangdong. Moreover, pseudoviruses generated in our laboratory with four civet S genes, each with a distinct RBD, infected cells expressing human receptor angiotensin-converting enzyme 2, but with 90 to 95% less efficiency compared to that of SARS-CoV. These four civet S genes were also constructed as DNA vaccines to immunize mice. Immunized sera elicited against most civet S glycoproteins displayed potent neutralizing activities against autologous viruses but were much less efficient (50% inhibitory concentration, 20- to 40-fold) at neutralizing SARS-CoV and vice versa. Convalescence-phase sera from humans were similarly ineffective against the dominant civet pseudovirus. Our findings suggest that the design of SARS vaccine should consider not only preventing the reemergence of SARS-CoV but also providing cross-protection, thus interrupting zoonotic transmission of a group of genetically divergent civet CoVs of broad geographic origin.
Figures
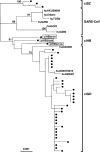
FIG. 1.
Phylogenetic neighbor-joining tree for civet-CoV S1 sequences. Three new Hubei strains are boxed. The reference sequences were obtained from GenBank. Human strains are spelled out, whereas civet viruses are presented as black dots. The GenBank accession numbers of the civet-CoVs include AY304486, AY304488 and -9, AY572034 and -5, AY572037 and -8, AY627044, AY627046 to 8, AY613948 and -9, AY613950 to 2, AY687354 and -6, AY687359, AY687360, AY686863 and -4, AY687361 to 9, and AY687370 and -2 (12, 23, 27). The horizontal branch is drawn in accordance with their relative genetic distances. The vertical lines provide clarity to the tree presentation. Bootstrap values of 1,000 replicates are labeled on the major branches. ci, civet; hu, human; SZ, Shenzen, GD, Guangdong; HB, Hubei.
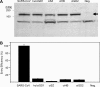
FIG. 2.
Expression and functional analysis of S glycoproteins derived from SARS-CoV and civet-CoVs. Similar levels of expression of each S glycoprotein (SARS-CoV, ciSZ, hu/ciGD1, ciGD2, or ciHB) are detected in Western blots stained with a polyclonal antibody to the first 400 amino acids of the N terminus (A). The arrow indicates the position of S glycoprotein. The vector cell lysate was used as a negative control. The viral entry efficiency was determined in human HEK293T-ACE2 cells (B). Five nanograms (measured by p24) of each pseudovirus was used to infect target cells. Luciferase activity was measured 72 h postinfection and normalized with SARS-CoV infection (100%). Five replicates were tested in each experiment. The average values and standard error bars are presented. The experiment was repeated three times.
FIG. 3.
Neutralization and cross-neutralization of SARS-CoV and civet-CoVs. Six groups of immune sera were tested against four civet pseudoviruses and an SARS-CoV pseudovirus. Five groups of sera were from mice immunized by electroporation with pcDNA3.1-OPT9 (top left panel), pcDNA3.1-ciSZ3-S (middle left panel), pcDNA3.1-ciHB-S (bottom left panel), pcDNA3.1-ciGD2-S (top right panel), and pcDNA3.1-hu/ciGD1-S (middle right panel) DNA vaccines, respectively. The sixth group of sera was from mice immunized with the ADS-MVA vaccine (bottom right panel). Sera were diluted from 1:100 to 1:72,900 and tested to neutralize 5 ng (measured by p24) of each pseudovirus in human HEK293T-ACE2 cells. Duplicates were tested in each experiment. The average values from each group are presented. Standard error bars are included. The experiment was repeated twice with similar results.
FIG. 4.
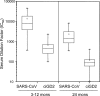
Resistance of ciGD2 pseudovirus to neutralization by human convalescent-phase sera of SARS patients. In comparison to the SARS-CoV pseudovirus, the ciGD2 pseudovirus displayed greatly reduced neutralization susceptibility to 20 human convalescent-phase sera collected 3 to 12 months p.r. at an average of 41-fold, which is highly significant (signed rank test; P < 0.005) (left panel). Ten additional serum samples collected at 24 months p.r. (right panel) show further reductions in NAb levels against both pseudoviruses. Consistently, ciGD2 pseudovirus displayed significantly less neutralization to these 10 convalescent-phase sera than did SARS-CoV pseudovirus, which is also highly significant (P < 0.005). The top and bottom of each rectangular box denote the 75th and 25th percentiles, respectively, with the median shown inside the box. Horizontal bars extending from each box represent the 99th and 1st percentiles. Duplicates were tested in each experiment. The average values are presented for each experiment. The experiment was repeated twice.
Similar articles
- Cross-neutralization of human and palm civet severe acute respiratory syndrome coronaviruses by antibodies targeting the receptor-binding domain of spike protein.
He Y, Li J, Li W, Lustigman S, Farzan M, Jiang S. He Y, et al. J Immunol. 2006 May 15;176(10):6085-92. doi: 10.4049/jimmunol.176.10.6085. J Immunol. 2006. PMID: 16670317 - Pathways of cross-species transmission of synthetically reconstructed zoonotic severe acute respiratory syndrome coronavirus.
Sheahan T, Rockx B, Donaldson E, Corti D, Baric R. Sheahan T, et al. J Virol. 2008 Sep;82(17):8721-32. doi: 10.1128/JVI.00818-08. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579604 Free PMC article. - Structural analysis of major species barriers between humans and palm civets for severe acute respiratory syndrome coronavirus infections.
Li F. Li F. J Virol. 2008 Jul;82(14):6984-91. doi: 10.1128/JVI.00442-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448527 Free PMC article. - Vaccine design for severe acute respiratory syndrome coronavirus.
He Y, Jiang S. He Y, et al. Viral Immunol. 2005;18(2):327-32. doi: 10.1089/vim.2005.18.327. Viral Immunol. 2005. PMID: 16035944 Review. - Molecular epidemiology, evolution and phylogeny of SARS coronavirus.
Luk HKH, Li X, Fung J, Lau SKP, Woo PCY. Luk HKH, et al. Infect Genet Evol. 2019 Jul;71:21-30. doi: 10.1016/j.meegid.2019.03.001. Epub 2019 Mar 4. Infect Genet Evol. 2019. PMID: 30844511 Free PMC article. Review.
Cited by
- SARS-CoV-2 Vaccine Development: Current Status.
Poland GA, Ovsyannikova IG, Crooke SN, Kennedy RB. Poland GA, et al. Mayo Clin Proc. 2020 Oct;95(10):2172-2188. doi: 10.1016/j.mayocp.2020.07.021. Epub 2020 Jul 30. Mayo Clin Proc. 2020. PMID: 33012348 Free PMC article. Review. - T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice.
Zhao J, Zhao J, Perlman S. Zhao J, et al. J Virol. 2010 Sep;84(18):9318-25. doi: 10.1128/JVI.01049-10. Epub 2010 Jul 7. J Virol. 2010. PMID: 20610717 Free PMC article. - Pseudotyped Viruses for Coronaviruses.
Wang M, Nie J, Wang Y. Wang M, et al. Adv Exp Med Biol. 2023;1407:133-151. doi: 10.1007/978-981-99-0113-5_7. Adv Exp Med Biol. 2023. PMID: 36920695 - Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission.
Graham RL, Baric RS. Graham RL, et al. J Virol. 2010 Apr;84(7):3134-46. doi: 10.1128/JVI.01394-09. Epub 2009 Nov 11. J Virol. 2010. PMID: 19906932 Free PMC article. Review. - Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus.
Wu K, Peng G, Wilken M, Geraghty RJ, Li F. Wu K, et al. J Biol Chem. 2012 Mar 16;287(12):8904-11. doi: 10.1074/jbc.M111.325803. Epub 2012 Jan 30. J Biol Chem. 2012. PMID: 22291007 Free PMC article.
References
- Centers for Disease Control and Prevention. 2003. Prevalence of IgG antibody to SARS-associated coronavirus in animal traders—Guangdong Province, China, 2003. Morb. Mortal. Wkly. Rep. 52:986-987. - PubMed
- Chen, Z., L. Zhang, C. Qin, L. Ba, C. E. Yi, F. Zhang, Q. Wei, T. He, W. Yu, J. Yu, H. Gao, X. Tu, A. Gettie, M. Farzan, K. Y. Yuen, and D. D. Ho. 2005. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J. Virol. 79:2678-2688. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous