Differential regulation of small heat shock proteins in transgenic mouse models of neurodegenerative diseases - PubMed (original) (raw)
Comparative Study
Differential regulation of small heat shock proteins in transgenic mouse models of neurodegenerative diseases
Jiou Wang et al. Neurobiol Aging. 2008 Apr.
Abstract
Previously, several studies have demonstrated changes in the levels of small heat shock proteins (sHSP) in the transgenic mouse models of familial amyotrophic lateral sclerosis (fALS) linked to mutations in Cu/Zn superoxide dismutase. Here, we compared the expression of sHSPs in transgenic mouse models of fALS, Parkinson's disease (PD), dentato-rubral pallido-luysian atrophy (DRPLA) and Huntington's disease (HD); where the expression of mutant cDNA genes was under the transcriptional regulation of the mouse prion protein promoter. These models express G37R mutant Cu/Zn superoxide dismutase (SOD1G37R; fALS), A53T mutant alpha-synuclein (alpha-SynA53T; PD), full-length mutant atrophin-1-65Q, and htt-N171-82Q (huntingtin N-terminal fragment; HD). We found that the levels and solubilities of two sHSPs, Hsp25 and alpha B-crystallin, were differentially regulated in these mice. Levels of both Hsp25 and alpha B-crystallin were markedly increased in subgroups of glias at the affected regions of symptomatic SODG37R and alpha-SynA53T transgenic mice; abnormal deposits or cells intensely positive for alpha B-crystallin were observed in SODG37R mice. By contrast, neither sHSP was induced in spinal cords of htt-N171-82Q or atrophin-1-65Q mice, which do not develop astrocytosis or major motor neuron abnormalities. Interestingly, the levels of insoluble alpha B-crystallin in spinal cords gradually increased as a function of age in nontransgenic animals. In vitro, alpha B-crystallin was capable of suppressing the aggregation of alpha-SynA53T, as previously described for a truncated mutant SOD1. The transgenes in these mice are expressed highly in astrocytes and thus our results suggest a role for small heat shock proteins in protecting activated glial cells such as astrocytes in neurodegenerative diseases.
Conflict of interest statement
Disclosure Statement: No current conflicts of interest are present between this work and any of the coauthors.
Figures
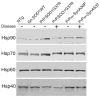
Fig. 1. The levels of catalytic chaperones (Hsp90, Hsp70, Hsp60, and Hsp40) do not change in disease associated with mutant SOD1 and mutant α-Syn
20 μg of protein from soluble fractions of the spinal cord homogenates were separated by SDS-PAGE followed by Western blotting with antibodies against Hsp90, Hsp70, Hsp60, or Hsp40. Samples include symptomatic (+) mice homozygous for PrP.SOD1G37R or hemizygous fro PrP.α-SynA53T, and non-symptomatic (−) age-matched controls including non-transgenic (NTg), those carrying the human wild-type genomic gene (Gn.SOD1WT), hemizygous for PrP.SOD1G37R, or hemizygous for PrP.α-SynA30P.
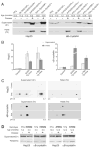
Fig. 2. Increased expression and altered solubility of Hsp25 and αB-crystallin associated with disease in mutant SOD1 and mutant α-Syn mouse models
(A) Spinal cord homogenates were extracted into the soluble fraction S1 and the non-ionic detergent insoluble fraction P2. 20 μg of S1 protein and 12 μg of P2 proteins were separated by SDS-PAGE followed by Western blotting with anti-Hsp25 or anti- αB-crystallin antibodies. For mouse genotype denotation, Gn denotes the genomic construct transgene, and PrP the mouse Prion promoter transgene; “+/+” denotes homozygous for the transgene, and “+” hemizygous. For disease phenotype, “+” denotes symptomatic or paralytic, and “−“ non-symptomatic. Note that PrP.α-Syn samples were duplicated in the anti-αB-crystallin blot to demonstrate the variability in P2 fractions. (B) Western analyses are summarized graphically. Controls were age-matched NTg, Gn.SOD1WT, or PrP.α-SynA30P mice, and their sHSP levels were normalized to 1. The relative levels of Hsp25 or αB-crystallin in symptomatic SOD1 mice (n = 6, three Gn.SOD1G37R and three PrP.SOD1G37R mice) or α-Syn mice (n = 6, PrP.α-SynA53T) were measured by comparing to controls on the same gel, and the fold increase from the control values were plotted (Means and standard errors of means). (C) 2D-PAGE analysis of Hsp25 and αB-crystallin. Soluble and insoluble spinal cord proteins from normal or sick Gn.SOD1G37R (line 29) mice were separated by 2D-PAGE (IEF/SDS-PAGE) and Western blotted for Hsp25 or αB-crystallin. Upper panels show the analyses of Hsp25. The three charged isoforms (likely representing different phosphorylation states) are proportionally increased in both the soluble and insoluble fractions from the affected tissues. Lower panels show the analyses of αB-crystallin. At least two isoelectric isoforms (likely representing phophorylation states) are proportionally increased in both the soluble and insoluble fractions. (D) Representative immunoblots of spinal cord tissues from symptomatic PrP.htt-N171-82Q (HD82Q) and PrP.AT65Q transgenic mice (40 μg of S1 protein and 20 μg of P2 proteins) with Hsp25 or αB-crystallin antibodies. “+” denotes disease phenotypes as characterized in previous studies [35;36].
Fig. 3. Up-regulation of insoluble αB-crystallin in the CNS during normal aging
(A) Normal male non-transgenic (NTg) mice with different ages from the same strain (C57BL/6L X C3H/HeJ) were used to collect their spinal cords, from which 20 μg of S1 protein and 12 μg of P2 protein were subjected to SDS-PAGE and Western blotting with an anti-αB-crystallin antibody and an anti-Hsp25 antibody. Immunoblot analysis for GAPDH was used as loading control. A symptomatic PrP.α-SynA53T mouse was used as comparison. (B) The relative levels of sHSPs were determined by densitometry from the immunoblots shown in panel A. The linear regression analysis clearly shows aging is associated with significant increase (i.e. slope is significantly different from 0) in sHSP expression (R2 and p values are: 0.9670 and 0.0026 for Hsp25, 0.8379 and 0.029 for soluble αB-crystallin, and 0.9846 and 0.0078 for insoluble αB-crystallin).
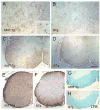
Fig. 4. Immunohistochemistry of Hsp25 and αB-crystallin in the PrP.SOD1G37R transgenic mice
Representative images from symptomatic mice (SOD-Tg, A, C, D, E, and G) and age-matched NTg mice (B, F, H, and I) immunostained for Hsp25 (A–D) or αB-crystallin (E–I). The Hsp25 staining of the cerebellar white matter (A, B) and a sagittal section of spinal cord (C, D) shows increased astroglial Hsp25 staining in SOD-Tg mice. Higher magnification image of spinal cord section (D) shows typical astrocyte staining (white arrow) and an inclusion body of abnormal cell (black arrow). Representative αB-crystallin stained sections of spinal cords from symptomatic mice (E, G) and age-matched NTg mice (B, H, I) are shown. Higher magnification images (G–I) show irregular- or round-shaped cell remnants or inclusion bodies in SOD-Tg mice (G) where as αB-crystallin staining is limited to cell bodies of apparent oligodendrocytes in NTg mice (H, I). Even heavier staining of NTg spinal cord (I) do not show aberrant staining seen in SOD-Tg mice. Scale bar: 50 μm for A, B, E & F; 20 μm for C, D, G-I. 4V, the fourth ventricle.
Fig. 5. Immunohistochemistry of Hsp25 in the PrP.α-SynA53T transgenic mice
Representative Hsp25 stained images from disease-affected mice (A, C) and age-matched NTg mice (B, D) are shown. Up-regulation of Hsp25 is limited to astrocytes but less profound than with the mutant SOD1 mice. Shown are sagittal sections of brain stem (A, B) and horizontal sections of spinal cord (C, D). GFAP staining of the spinal cord sections (E, F) shows severe astrogliosis in A53T Tg mice. Luxol Fast Blue (LFB) staining of spinal cord (G, H) also shows distintegration of myelinated fiber tracts in A53T Tg mice. Scale bar: 50 μm for A, B; 200 μm for C, D, G, H; 250 μm for E, F.
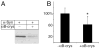
Fig. 6. αB-crystallin suppresses aggregation of α-Syn
Brain supernatants from young α-SynA53T transgenic mice, containing 1 μg/μl total protein, with 0.4 μg/ul of BSA (A) or 0.4 μg/ul of purified α-crystallin (predominantly αB-crystallin) (B), were incubated at 37°C with shaking for 12 hrs and centrifuged to collect the pellet fraction containing aggregated proteins. The pellet fraction was analyzed by Western blotting with an anti-α-Syn antibody. Adding α-crystallin significantly suppresses the aggregation of α-Syn (B). Asterisk marks significance with one-tailed p < 0.01 in a Student’s t-test; n = 5; error bars represent standard deviations.
Similar articles
- Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: alphaB-crystallin modulates aggregation.
Wang J, Xu G, Li H, Gonzales V, Fromholt D, Karch C, Copeland NG, Jenkins NA, Borchelt DR. Wang J, et al. Hum Mol Genet. 2005 Aug 15;14(16):2335-47. doi: 10.1093/hmg/ddi236. Epub 2005 Jul 6. Hum Mol Genet. 2005. PMID: 16000321 - Oxidative stress, mutant SOD1, and neurofilament pathology in transgenic mouse models of human motor neuron disease.
Tu PH, Gurney ME, Julien JP, Lee VM, Trojanowski JQ. Tu PH, et al. Lab Invest. 1997 Apr;76(4):441-56. Lab Invest. 1997. PMID: 9111507 Review. - The small heat shock proteins, especially HspB4 and HspB5 are promising protectants in neurodegenerative diseases.
Zhu Z, Reiser G. Zhu Z, et al. Neurochem Int. 2018 May;115:69-79. doi: 10.1016/j.neuint.2018.02.006. Epub 2018 Feb 7. Neurochem Int. 2018. PMID: 29425965 Review. - Environmental, pharmacological, and genetic modulation of the HD phenotype in transgenic mice.
Schilling G, Savonenko AV, Coonfield ML, Morton JL, Vorovich E, Gale A, Neslon C, Chan N, Eaton M, Fromholt D, Ross CA, Borchelt DR. Schilling G, et al. Exp Neurol. 2004 May;187(1):137-49. doi: 10.1016/j.expneurol.2004.01.003. Exp Neurol. 2004. PMID: 15081595 - Relationship of oxygen radical-induced lipid peroxidative damage to disease onset and progression in a transgenic model of familial ALS.
Hall ED, Andrus PK, Oostveen JA, Fleck TJ, Gurney ME. Hall ED, et al. J Neurosci Res. 1998 Jul 1;53(1):66-77. doi: 10.1002/(SICI)1097-4547(19980701)53:1<66::AID-JNR7>3.0.CO;2-H. J Neurosci Res. 1998. PMID: 9670993
Cited by
- Aberrant CHCHD2-associated mitochondriopathy in Kii ALS/PDC astrocytes.
Leventoux N, Morimoto S, Ishikawa M, Nakamura S, Ozawa F, Kobayashi R, Watanabe H, Supakul S, Okamoto S, Zhou Z, Kobayashi H, Kato C, Hirokawa Y, Aiba I, Takahashi S, Shibata S, Takao M, Yoshida M, Endo F, Yamanaka K, Kokubo Y, Okano H. Leventoux N, et al. Acta Neuropathol. 2024 May 15;147(1):84. doi: 10.1007/s00401-024-02734-w. Acta Neuropathol. 2024. PMID: 38750212 - The heat shock response in neurons and astroglia and its role in neurodegenerative diseases.
San Gil R, Ooi L, Yerbury JJ, Ecroyd H. San Gil R, et al. Mol Neurodegener. 2017 Sep 18;12(1):65. doi: 10.1186/s13024-017-0208-6. Mol Neurodegener. 2017. PMID: 28923065 Free PMC article. Review. - α-Synucleinopathy associated c-Abl activation causes p53-dependent autophagy impairment.
Karim MR, Liao EE, Kim J, Meints J, Martinez HM, Pletnikova O, Troncoso JC, Lee MK. Karim MR, et al. Mol Neurodegener. 2020 Apr 16;15(1):27. doi: 10.1186/s13024-020-00364-w. Mol Neurodegener. 2020. PMID: 32299471 Free PMC article. - Structural and biophysical properties of the pathogenic SOD1 variant H46R/H48Q.
Winkler DD, Schuermann JP, Cao X, Holloway SP, Borchelt DR, Carroll MC, Proescher JB, Culotta VC, Hart PJ. Winkler DD, et al. Biochemistry. 2009 Apr 21;48(15):3436-47. doi: 10.1021/bi8021735. Biochemistry. 2009. PMID: 19227972 Free PMC article. - Zeta-crystallin: a moonlighting player in cancer.
Lulli M, Nencioni D, Papucci L, Schiavone N. Lulli M, et al. Cell Mol Life Sci. 2020 Mar;77(6):965-976. doi: 10.1007/s00018-019-03301-3. Epub 2019 Sep 28. Cell Mol Life Sci. 2020. PMID: 31563996 Free PMC article. Review.
References
- Adachi H, Katsuno M, Minamiyama M, Sang C, Pagoulatos G, Angelidis C, Kusakabe M, Yoshiki A, Kobayashi Y, Doyu M, Sobue G. Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J Neurosci. 2003;23:2203–2211. - PMC - PubMed
- Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Constitutive expression of heat shock protein HSP25 in the central nervous system of the developing and adult mouse. J Comp Neurol. 2001;434:262–274. - PubMed
- Benndorf R, Kraft R, Otto A, Stahl J, Bohm H, Bielka H. Purification of the growth-related protein p25 of the Ehrlich ascites tumor and analysis of its isoforms. Biochem Int. 1988;17:225–234. - PubMed
- Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal (Biomed Eng ) 1996;13:159–163. - PubMed
- Bruening W, Roy J, Giasson B, Figlewicz DA, Mushynski WE, Durham HD. Up-regulation of protein chaperones preserves viability of cells expressing toxic Cu/Zn-superoxide dismutase mutants associated with amyotrophic lateral sclerosis. J Neurochem. 1999;72:693–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS38065/NS/NINDS NIH HHS/United States
- NS38377/NS/NINDS NIH HHS/United States
- R01 NS038065-06/NS/NINDS NIH HHS/United States
- R01 NS038065-07/NS/NINDS NIH HHS/United States
- NS044278/NS/NINDS NIH HHS/United States
- R01 NS038065-05A2/NS/NINDS NIH HHS/United States
- P50 NS038377-080003/NS/NINDS NIH HHS/United States
- P50 NS038377-070003/NS/NINDS NIH HHS/United States
- R01 NS038065/NS/NINDS NIH HHS/United States
- R01 NS044278/NS/NINDS NIH HHS/United States
- P01 NS038065/NS/NINDS NIH HHS/United States
- R56 NS038065/NS/NINDS NIH HHS/United States
- P50 NS038377-06A10003/NS/NINDS NIH HHS/United States
- P50 NS038377/NS/NINDS NIH HHS/United States
- R01 NS 044278/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous