The Bcl-2 apoptotic switch in cancer development and therapy - PubMed (original) (raw)
Review
The Bcl-2 apoptotic switch in cancer development and therapy
J M Adams et al. Oncogene. 2007.
Abstract
Impaired apoptosis is both critical in cancer development and a major barrier to effective treatment. In response to diverse intracellular damage signals, including those evoked by cancer therapy, the cell's decision to undergo apoptosis is determined by interactions between three factions of the Bcl-2 protein family. The damage signals are transduced by the diverse 'BH3-only' proteins, distinguished by the BH3 domain used to engage their pro-survival relatives: Bcl-2, Bcl-x(L), Bcl-w, Mcl-1 and A1. This interaction ablates pro-survival function and allows activation of Bax and Bak, which commit the cell to apoptosis by permeabilizing the outer membrane of the mitochondrion. Certain BH3-only proteins (e.g. Bim, Puma) can engage all the pro-survival proteins, but others (e.g. Bad, Noxa) engage only subsets. Activation of Bax and Bak appears to require that the BH3-only proteins engage the multiple pro-survival proteins guarding Bax and Bak, rather than binding to the latter. The balance between the pro-survival proteins and their BH3 ligands regulates tissue homeostasis, and either overexpression of a pro-survival family member or loss of a proapoptotic relative can be oncogenic. Better understanding of the Bcl-2 family is clarifying its role in cancer development, revealing how conventional therapy works and stimulating the search for "BH3 mimetics" as a novel class of anticancer drugs.
Figures
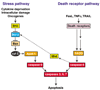
Figure 1
Pathways to cell death. The stress pathway is initiated by BH3-only proteins (‘BH3’), which inactivate the Bcl-2-like proteins, keeping them from restraining Bax and Bak. Bax or Bak can permeabilize the mitochondrial outer membrane, releasing cyto-chrome c, which provokes Apaf-1 (apoptotic protease-activating factor 1) to activate caspase-9. The ‘death receptor’ pathway is activated when ligands of the TNF family engage with and aggregate their cognate receptors on the cell surface and activate caspase-8 via adaptor proteins that include FADD. The two pathways are largely independent, but in certain cells the death receptor pathway engages the stress pathway via a cleaved form of the BH3-only protein Bid (tBid), which can engage Bcl-2 homologs and perhaps Bax (see text).
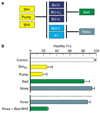
Figure 2
Differing binding profiles and apoptotic potency of BH3-only proteins. (a) The ability of Bim, Puma and tBid to engage all the pro-survival proteins contrasts with the selective binding of others, such as Bad and Noxa (Chen et al., 2005). (b) Cytotoxicity assays in fibroblasts show that Bim and Puma are potent killers, but Bad and Noxa alone are much weaker. Nevertheless, Noxa in conjunction with a Bad BH3 (within an inert backbone) kills potently (Chen et al., 2005).
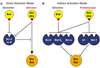
Figure 3
Contrasting direct and indirect activation models for Bax and Bak. (a) In the direct model (Letai et al., 2002), the putative activators Bim and tBid bind directly to Bax and Bak to drive their activation, whereas the sensitizers only bind to the pro-survival Bcl-2 homologs (‘Bcl-2 _et al._’) via the BH3 domain (red triangle). (b) In the indirect activation model (Chen et al., 2005; Willis et al., 2005, 2007), the BH3-only proteins activate Bax and Bak not by binding to them directly, but instead by engaging the multiple pro-survival proteins that guard Bax and Bak. In this model, Bim and tBid are more potent than Bad and other BH3-only proteins owing to the greater range of pro-survival proteins that they can engage and neutralize.
Figure 4
Model for the regulation of (a) Bak and (b) Bax by their pro-survival relatives. (a) In the healthy cell, both Bcl-xL and Mcl-1 can bind to Bak, presumably to a ‘primed’ conformer with its BH3 (red) exposed (Willis et al., 2007). Apoptosis is induced if and only if BH3-only proteins displace Bak from both these pro-survival guards. The free primed Bak may nucleate formation of the Bak oligomers (of unknown structure) that elicit permeabilization of the mitochondrial outer membrane and release of cytochrome c. (b) Bax appears to be regulated analogously (Willis et al., 2007), but most of the ‘unprimed’ Bax is cytosolic, and all the pro-survival family members (‘Bcl-2 _et al_’) appear to inhibit Bax activation. Whether a small amount of the postulated primed Bak and Bax exists in healthy cells, perhaps on the mitochondrial membrane, or instead is formed early in apoptosis by an unknown signal, is not yet established. The structures of the complexes is not known, and the integration of the proteins into the membrane probably includes regions in addition to their C-terminal regions (see text).
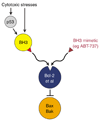
Figure 5
Potential of BH3 mimetic anticancer drugs. Because the upstream signals that induce apoptosis (particularly the p53 pathway) often are compromised in cancer cells, a drug that behaves like a BH3-only protein by binding to one or more of the Bcl-2 homologs to free Bax or Bak should be a more effective way of killing cancer cells.
Similar articles
- Subversion of the Bcl-2 life/death switch in cancer development and therapy.
Adams JM, Huang DC, Strasser A, Willis S, Chen L, Wei A, van Delft M, Fletcher JI, Puthalakath H, Kuroda J, Michalak EM, Kelly PN, Bouillet P, Villunger A, O'Reilly L, Bath ML, Smith DP, Egle A, Harris AW, Hinds M, Colman P, Cory S. Adams JM, et al. Cold Spring Harb Symp Quant Biol. 2005;70:469-77. doi: 10.1101/sqb.2005.70.009. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869785 - The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner.
Kazi A, Sun J, Doi K, Sung SS, Takahashi Y, Yin H, Rodriguez JM, Becerril J, Berndt N, Hamilton AD, Wang HG, Sebti SM. Kazi A, et al. J Biol Chem. 2011 Mar 18;286(11):9382-92. doi: 10.1074/jbc.M110.203638. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148306 Free PMC article. - Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins.
Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Willis SN, et al. Genes Dev. 2005 Jun 1;19(11):1294-305. doi: 10.1101/gad.1304105. Epub 2005 May 18. Genes Dev. 2005. PMID: 15901672 Free PMC article. - Life in the balance: how BH3-only proteins induce apoptosis.
Willis SN, Adams JM. Willis SN, et al. Curr Opin Cell Biol. 2005 Dec;17(6):617-25. doi: 10.1016/j.ceb.2005.10.001. Epub 2005 Oct 21. Curr Opin Cell Biol. 2005. PMID: 16243507 Free PMC article. Review. - BAD: undertaker by night, candyman by day.
Danial NN. Danial NN. Oncogene. 2008 Dec;27 Suppl 1:S53-70. doi: 10.1038/onc.2009.44. Oncogene. 2008. PMID: 19641507 Review.
Cited by
- BAD overexpression inhibits cell growth and induces apoptosis via mitochondrial-dependent pathway in non-small cell lung cancer.
Jiang L, Luo M, Liu D, Chen B, Zhang W, Mai L, Zeng J, Huang N, Huang Y, Mo X, Li W. Jiang L, et al. Cancer Cell Int. 2013 Jun 1;13(1):53. doi: 10.1186/1475-2867-13-53. Cancer Cell Int. 2013. PMID: 23725574 Free PMC article. - Association between Virulence Factors and TRAF1/4-1BB/Bcl-xL Expression in Gastric Mucosa Infected with Helicobacter pylori.
Wang F, Wu X, Liu Z, Bu G, Li X, Qu N, Peng J, Xu C, Shen S, Yuan Y. Wang F, et al. Gastroenterol Res Pract. 2015;2015:648479. doi: 10.1155/2015/648479. Epub 2015 Feb 8. Gastroenterol Res Pract. 2015. PMID: 25737718 Free PMC article. - The role of autophagy in metal-induced urogenital carcinogenesis.
Saran U, Tyagi A, Chandrasekaran B, Ankem MK, Damodaran C. Saran U, et al. Semin Cancer Biol. 2021 Nov;76:247-257. doi: 10.1016/j.semcancer.2021.03.022. Epub 2021 Mar 30. Semin Cancer Biol. 2021. PMID: 33798723 Free PMC article. Review. - Anti-Cancer Activities of Nano Amorphous Calcium Phosphates toward Premalignant and Oral Cancer Cells.
Herendija E, Jakšić Karišik M, Milašin J, Lazarević M, Ignjatović N. Herendija E, et al. Biomedicines. 2024 Jul 5;12(7):1499. doi: 10.3390/biomedicines12071499. Biomedicines. 2024. PMID: 39062071 Free PMC article. - KUD773, a phenylthiazole derivative, displays anticancer activity in human hormone-refractory prostate cancers through inhibition of tubulin polymerization and anti-Aurora A activity.
Yu CC, Liu SP, Hsu JL, Hsu JT, Kudryavtsev KV, Guh JH. Yu CC, et al. J Biomed Sci. 2015 Jan 7;22(1):2. doi: 10.1186/s12929-014-0107-x. J Biomed Sci. 2015. PMID: 25563361 Free PMC article.
References
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. - PubMed
- Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. - PubMed
- Bellot G, Cartron PF, Er E, Oliver L, Juin P, Armstrong LC, et al. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 2006 [Epub ahead of print] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials