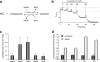Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells - PubMed (original) (raw)
Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells
Elaine D MacKenzie et al. Mol Cell Biol. 2007 May.
Abstract
Succinate dehydrogenase (SDH) and fumarate hydratase (FH) are components of the tricarboxylic acid (TCA) cycle and tumor suppressors. Loss of SDH or FH induces pseudohypoxia, a major tumor-supporting event, which is the activation of hypoxia-inducible factor (HIF) under normoxia. In SDH- or FH-deficient cells, HIF activation is due to HIF1alpha stabilization by succinate or fumarate, respectively, either of which, when in excess, inhibits HIFalpha prolyl hydroxylase (PHD). To reactivate PHD, we focused on its substrate, alpha-ketoglutarate. We designed and synthesized cell-permeating alpha-ketoglutarate derivatives, which build up rapidly and preferentially in cells with a dysfunctional TCA cycle. This study shows that succinate- or fumarate-mediated inhibition of PHD is competitive and is reversed by pharmacologically elevating intracellular alpha-ketoglutarate. Introduction of alpha-ketoglutarate derivatives restores normal PHD activity and HIF1alpha levels to SDH-suppressed cells, indicating new therapy possibilities for the cancers associated with TCA cycle dysfunction.
Figures
FIG. 1.
Succinate-mediated inhibition of PHD can be overcome by increasing α-ketoglutarate levels in vitro. A hydroxylation reaction of the ODD domain was carried out in vitro with the indicated amounts of succinate and α-ketoglutarate (αKG). Hydroxylation of ODD (OH-ODD) resulted in a faster-migrating band on SDS-PAGE.
FIG. 2.
The intracellular α-ketoglutarate level is elevated in cells treated with the α-ketoglutarate ester derivatives. (a) Schematic illustration of the GDH reaction. (b) The decrease in NADH (as measured by light absorbance at 340 nm) is stoichiometric to α-ketoglutarate added to the GDH reaction. (c) HEK293 cells were either left untreated or treated with 1 mM of the indicated α-ketoglutarate derivative or with underivatized α-ketoglutarate. The α-ketoglutarate level in cell extracts was analyzed using the GDH reaction as in panel b. (d) HEK293 cells were transfected with either the control Sc or shRNAs targeting SDHD (Di3 or Di4) and 48 h later cells were either left untreated or treated with 1 mM octyl-α-ketoglutarate. Intracellular levels of α-ketoglutarate were analyzed as in panels b and c. aKG, underivatized α-ketoglutarate; OaKG, TaKG, and BaKG, octyl-, TFMB-, and benzyl-α-ketoglutarate esters, respectively. The error bars indicate standard deviations.
FIG. 3.
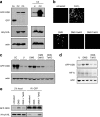
The inhibition of PHD activity by succinate in cells is alleviated by the increase in the intracellular α-ketoglutarate level. (a, left) Clones (C2 and C3) coexpressing the GFP-ODD fusion protein and HA-tagged pVHL were analyzed by Western blotting. Cells transiently transfected with a plasmid encoding GFP alone were used as a reference for GFP molecular weight (Co). Actin was used as a loading control. (Right) Clone 3 (C3) cells were either left untreated (U) or treated with the hypoxia-mimetic compound CoCl2 (CC), and GFP-ODD and HA-pVHL protein levels were analyzed by Western blotting. (b) Clone 3 cells were either left untreated or treated with CoCl2 or 25 mM dimethyl-succinate (DMS) for 48 h. Where indicated, 1 mM of the α-ketoglutarate derivatives was added for the final 12 h of the incubation (with dimethyl-succinate) and GFP-ODD levels were visualized microscopically. (c) Clone 3 cells were treated as in panel b, and GFP-ODD levels were detected by Western blotting. CoCl2-treated cells (CC) were used as a positive control for PHD inhibition, and the actin level was used as a loading control. (d) Clone 3 cells were either left untreated (U) or treated with dimethyl-succinate (DMS) with or without TFMB-α-ketoglutarate. GFP-ODD and HIF1α levels were detected by Western blotting. Actin was used as a loading control. (e) Clone 3 cells were treated as in panel d, and protein lysate was immunoprecipitated using anti-GFP (polyclonal) antibody. The lysate before (2% input) or after immunoprecipitation (IP) was analyzed by Western blotting using anti-GFP (monoclonal) antibody and anti-HA antibody. OaKG and TaKG, octyl- and TFMB-α-ketoglutarate esters, respectively.
FIG. 4.
α-Ketoglutarate esters overcome HIF1α stabilization mediated by succinate or fumarate. (a) HEK293 cells were transfected with either scrambled control Sc or shRNA targeting SDHD (Di3). Where indicated, the α-ketoglutarate derivatives were added 48 h after transfection, followed by a Western blot analysis for HIF1α. The actin level was used as a loading control. (b) HCT116 cells were treated with or without 2 mM monoethyl-fumarate for 24 h and/or 2 mM TFMB-α-ketoglutarate ester for the last 2 h prior to extraction. Western blot analysis was performed as in panel a. (c) ARPE cells were either left untreated or treated with the indicated amounts of monoethyl-fumarate for 3 h, with or without 2 mM octyl-α-ketoglutarate ester. Western blot analysis was performed as in panel a. (d) HEK293 cells were left untreated or treated with 0.5 mM CoCl2 for 2 h with or without 2 mM of the indicated α-ketoglutarate derivatives. Western blot analysis was performed as in panel a. (e) ARPE cells were either untreated or treated with 2 mM monoethyl-fumarate and/or 2 mM TFMB-α-ketoglutarate ester. The endogenous protein levels of HIF1α, HIF2α, pVHL, and actin were detected by Western blotting. U, untreated; CC, CoCl2; MEF, monoethyl-fumarate; OaKG, octyl-α-ketoglutarate; TaKG, TFMB-α-ketoglutarate; BaKG, benzyl-α-ketoglutarate.
FIG. 5.
α-Ketoglutarate retargets HIF1α for ubiquitylation and proteasomally mediated degradation. (a) ARPE cells were left untreated or treated with 2 mM monoethyl-fumarate for 24 h with or without 2 mM of octyl-α-ketoglutarate ester added 30 min before cell lysis. Where indicated, MG132, a proteasomal inhibitor, was added to cells 30 min prior to the α-ketoglutarate derivative. HIF1α and actin as loading control were analyzed by Western blotting. (b) Parental RCC4 cells (VHL negative) of _VHL_-transfected RCC4 cells were either left untreated or treated with 2 mM monoethyl-fumarate with or without 2 mM TFMB-α-ketoglutarate as indicated. HIF1α and actin as loading controls were analyzed by Western blotting. (c) ARPE cells were either untreated or treated with 2 mM monoethyl fumarate with or without TFMB-α-ketoglutarate ester for 12 h. The glycolytic rate was analyzed as described in Materials and Methods. U, untreated; MEF, monoethyl-fumarate; OaKG, octyl-α-ketoglutarate; TaKG, TFMB-α-ketoglutarate.
Similar articles
- Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors.
Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, Zhao S, Ye D, Xiong Y, Guan KL. Xiao M, et al. Genes Dev. 2012 Jun 15;26(12):1326-38. doi: 10.1101/gad.191056.112. Epub 2012 Jun 7. Genes Dev. 2012. PMID: 22677546 Free PMC article. - Intermediary metabolite precursor dimethyl-2-ketoglutarate stabilizes hypoxia-inducible factor-1α by inhibiting prolyl-4-hydroxylase PHD2.
Hou P, Kuo CY, Cheng CT, Liou JP, Ann DK, Chen Q. Hou P, et al. PLoS One. 2014 Nov 24;9(11):e113865. doi: 10.1371/journal.pone.0113865. eCollection 2014. PLoS One. 2014. PMID: 25420025 Free PMC article. - Redox stress is not essential for the pseudo-hypoxic phenotype of succinate dehydrogenase deficient cells.
Selak MA, Durán RV, Gottlieb E. Selak MA, et al. Biochim Biophys Acta. 2006 May-Jun;1757(5-6):567-72. doi: 10.1016/j.bbabio.2006.05.015. Epub 2006 May 17. Biochim Biophys Acta. 2006. PMID: 16797480 - Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer.
King A, Selak MA, Gottlieb E. King A, et al. Oncogene. 2006 Aug 7;25(34):4675-82. doi: 10.1038/sj.onc.1209594. Oncogene. 2006. PMID: 16892081 Review. - The connection between tricarboxylic acid cycle enzyme mutations and pseudohypoxic signaling in pheochromocytoma and paraganglioma.
Wang Y, Liu B, Li F, Zhang Y, Gao X, Wang Y, Zhou H. Wang Y, et al. Front Endocrinol (Lausanne). 2023 Oct 5;14:1274239. doi: 10.3389/fendo.2023.1274239. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37867526 Free PMC article. Review.
Cited by
- Long-Noncoding RNA (lncRNA) in the Regulation of Hypoxia-Inducible Factor (HIF) in Cancer.
Barth DA, Prinz F, Teppan J, Jonas K, Klec C, Pichler M. Barth DA, et al. Noncoding RNA. 2020 Jul 6;6(3):27. doi: 10.3390/ncrna6030027. Noncoding RNA. 2020. PMID: 32640630 Free PMC article. Review. - Linking metabolism and epigenetic regulation in development of hepatocellular carcinoma.
Puszyk WM, Trinh TL, Chapple SJ, Liu C. Puszyk WM, et al. Lab Invest. 2013 Sep;93(9):983-90. doi: 10.1038/labinvest.2013.94. Epub 2013 Aug 5. Lab Invest. 2013. PMID: 23917878 Free PMC article. Review. - Aglycemic HepG2 Cells Switch From Aminotransferase Glutaminolytic Pathway of Pyruvate Utilization to Complete Krebs Cycle at Hypoxia.
Ježek J, Plecitá-Hlavatá L, Ježek P. Ježek J, et al. Front Endocrinol (Lausanne). 2018 Oct 26;9:637. doi: 10.3389/fendo.2018.00637. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30416487 Free PMC article. - Defining a metabolic landscape of tumours: genome meets metabolism.
Seth Nanda C, Venkateswaran SV, Patani N, Yuneva M. Seth Nanda C, et al. Br J Cancer. 2020 Jan;122(2):136-149. doi: 10.1038/s41416-019-0663-7. Epub 2019 Dec 10. Br J Cancer. 2020. PMID: 31819196 Free PMC article. Review. - miR-147b-mediated TCA cycle dysfunction and pseudohypoxia initiate drug tolerance to EGFR inhibitors in lung adenocarcinoma.
Zhang WC, Wells JM, Chow KH, Huang H, Yuan M, Saxena T, Melnick MA, Politi K, Asara JM, Costa DB, Bult CJ, Slack FJ. Zhang WC, et al. Nat Metab. 2019 Apr;1(4):460-474. doi: 10.1038/s42255-019-0052-9. Epub 2019 Apr 8. Nat Metab. 2019. PMID: 31535082 Free PMC article.
References
- Astrom, K., J. E. Cohen, J. E. Willett-Brozick, C. E. Aston, and B. E. Baysal. 2003. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum. Genet. 113:228-237. - PubMed
- Bensaad, K., A. Tsuruta, M. A. Selak, M. N. Vidal, K. Nakano, R. Bartrons, E. Gottlieb, and K. H. Vousden. 2006. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126:107-120. - PubMed
- Covello, K. L., and M. C. Simon. 2004. HIFs, hypoxia, and vascular development. Curr. Top. Dev. Biol. 62:37-54. - PubMed
- Dahia, P. L. M., K. N. Ross, M. E. Wright, C. Y. Hayashida, S. Santagata, M. Barontini, A. L. Kung, G. Sanso, J. F. Powers, A. S. Tischler, R. Hodin, S. Heitritter, F. J. Moore, R. Dluhy, J. A. Sosa, I. T. Ocal, D. E. Benn, D. J. Marsh, B. G. Robinson, K. Schneider, J. Garber, S. M. Arum, M. Korbonits, A. Grossman, P. Pigny, S. P. A. Toledo, V. Nose, C. Li, and C. D. Stiles. 2005. A HIF1α regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 1:e8. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous