Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head - PubMed (original) (raw)
Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head
Gülgün Tezel et al. Invest Ophthalmol Vis Sci. 2007 Mar.
Abstract
Purpose: This study aimed to determine the association between advanced glycation end products (AGEs) and glaucoma based on the known synergism between oxidative stress with AGEs and the evidence of oxidative stress during glaucomatous neurodegeneration.
Methods: The extent and cellular localization of immunolabeling for AGEs and their receptor, RAGE, were determined in histologic sections of the retina and optic nerve head obtained from 38 donor eyes with glaucoma and 30 eyes from age-matched donors without glaucoma.
Results: The extent of AGE and RAGE immunolabeling was greater in older than in younger donor eyes. However, compared with age-matched controls, an enhanced accumulation of AGEs and an up-regulation of RAGE were detectable in the glaucomatous retina and optic nerve head. Although some retinal ganglion cells (RGCs) and glia exhibited intracellular immunolabeling for AGEs, increased AGE immunolabeling in glaucomatous eyes was predominantly extracellular and included laminar cribriform plates in the optic nerve head. Some RAGE immunolabeling was detectable on RGCs; however, increased RAGE immunolabeling in glaucomatous eyes was predominant on glial cells, primarily Müller cells.
Conclusions: Given that the generation of AGEs is an age-dependent event, increased AGE accumulation in glaucomatous tissues supports that an accelerated aging process accompanies neurodegeneration in glaucomatous eyes. One of the potential consequences of AGE accumulation in glaucomatous eyes appears to be its contribution to increased rigidity of the lamina cribrosa. The presence of RAGE on RGCs and glia also makes them susceptible to AGE-mediated events through receptor-mediated signaling, which may promote cell death or dysfunction during glaucomatous neurodegeneration.
Figures
FIGURE 1
The intensity of immunolabeling for AGEs and RAGE was first qualitatively graded as negative (−), faint (+), moderate (++), and strong (+++). Immunolabeling was then quantitatively graded on digitized images in a masked fashion by measuring the specific immunolabeling areas. A percentage value was expressed for each slide relative to the average ratio of the total area analyzed. Mean values are presented. Immunolabeling for AGEs and RAGE were qualitatively and quantitatively greater in the eyes of donors with glaucoma than in those of age-matched controls. Age-matched control data shown do not include four control eyes of donors younger than 55 years for appropriate age matching (38 glaucomatous vs. 26 control eyes).
FIGURE 2
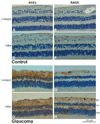
Immunoperoxidase labeling of the retina for AGEs and RAGE. (A, B) Histologic retinal sections obtained from a 55-year-old and a 94-year-old donor without glaucoma, respectively (donors 5 and 27). The extent of AGE immunolabeling was greater in older than in younger eyes. (C, D) AGE immunolabeling of retinal sections obtained from moderately damaged glaucomatous eyes (donors 1 and 34). Retinal immunolabeling for AGEs was more prominent in eyes of donors with glaucoma than in age-matched controls. Note the prominent AGE immunolabeling in the inner limiting membrane and around retinal blood vessels (v). Scattered cells in the RGC layer exhibited some immunolabeling for AGEs. Increased AGE immunolabeling in the glaucomatous retina was also determined to be associated with extracellular structures. (E, F) RAGE immunolabeling in the control retinas presented in (A) and (B), respectively. Similar to AGE immunolabeling, retinal immunoperoxidase labeling for RAGE was greater in older than in younger eyes. (G, H) RAGE immunolabeling in the glaucomatous retinas presented in (C) and (D), respectively. Retinal immunolabeling for RAGE was greater in eyes of donors with glaucoma than in those of age-matched controls. Increased RAGE immunolabeling in the glaucomatous retina was predominantly associated with the retinal distribution of Müller cells. Some RAGE immunolabeling was also detectable on scattered cells in the RGC layer and around blood vessels. Chromagen, DAB; nuclear counterstain, Mayer hematoxylin; gc, ganglion cell; in, inner nuclear; on, outer nuclear layers; v, blood vessel).
FIGURE 3
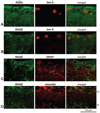
Double-immunofluorescence labeling of the glaucomatous retina. (A) Double-immunofluorescence labeling for AGEs (green) and brn-3 (red). (B–D) Double immunolabeling for RAGE (green) and brn-3, GFAP, and vimentin, respectively (red). Images were obtained from a moderately damaged glaucomatous eye (donor 22). Brn-3-positive RGCs exhibited some granular intracellular immunolabeling for AGEs (A, yellow). However, AGE immunolabeling in the glaucomatous retina was also associated with glial cells and extracellular components. Increased RAGE immunolabeling was detectable on some brn-3-positive RGCs and GFAP-positive astrocytes (B, C, yellow). However, increased RAGE immunolabeling in the glaucomatous retina was predominantly localized to vimentin-positive Müller cells (D, yellow). gc, ganglion cell; in, inner nuclear layer.
FIGURE 4
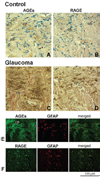
Optic nerve head immunolabeling for AGEs and RAGE. (A, B) Immunoperoxidase labeling of the control optic nerve head (donor 21) for AGEs and RAGE, respectively. (C, D) Immunoperoxidase labeling of the glaucomatous optic nerve head (donor 25) for AGEs and RAGE, respectively. AGE and RAGE immunolabeling were greater in the eyes of donors with glaucoma than in those of age-matched controls (chromagen, DAB; nuclear counterstain, Mayer hematoxylin). Note the prominent immunolabeling of nerve bundles (nb) for AGEs in panel C. (E) Double-immunofluorescence labeling of the glaucomatous optic nerve head for AGEs (green) and GFAP (red). (F) Double-immunofluorescence labeling of the glaucomatous optic nerve head for RAGE (green) and GFAP (red). Increased AGE immunolabeling in the glaucomatous optic nerve head was mostly extracellular in the cribriform plates (cp) of the lamina cribrosa. Some GFAP-positive astrocytes (E, yellow) and nerve bundles were also positive for AGE immunolabeling. However, increased RAGE immunolabeling was mainly localized to GFAP-positive astrocytes in the glaucomatous optic nerve head (F, yellow). A Blood vessel (v) also exhibits immunolabeling for AGEs in panel E.
FIGURE 5
Double-immunofluorescence labeling of the glaucomatous retina. (A) Double-immunofluorescence labeling of the glaucomatous retina for AGEs (green) and DNP (red). (B) Double-immunofluorescence labeling of the glaucomatous optic nerve head for AGEs (green) and DNP (red). Images were obtained from a moderately damaged glaucomatous donor eye (donor 12). DNP immunolabeling for protein carbonyls was predominant in the inner retinal layers and colocalized (yellow) with immunolabeling for AGEs. gc, ganglion cell; in, inner nuclear; on, outer nuclear layers; cp, cribriform plates of the lamina cribrosa.
Similar articles
- Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes.
Tezel G, Hernandez R, Wax MB. Tezel G, et al. Arch Ophthalmol. 2000 Apr;118(4):511-8. doi: 10.1001/archopht.118.4.511. Arch Ophthalmol. 2000. PMID: 10766137 - Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head.
Tezel G, Wax MB. Tezel G, et al. Arch Ophthalmol. 2004 Sep;122(9):1348-56. doi: 10.1001/archopht.122.9.1348. Arch Ophthalmol. 2004. PMID: 15364715 - Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma.
Neufeld AH. Neufeld AH. Arch Ophthalmol. 1999 Aug;117(8):1050-6. doi: 10.1001/archopht.117.8.1050. Arch Ophthalmol. 1999. PMID: 10448748 - The pathogenic role of transforming growth factor-β2 in glaucomatous damage to the optic nerve head.
Fuchshofer R. Fuchshofer R. Exp Eye Res. 2011 Aug;93(2):165-9. doi: 10.1016/j.exer.2010.07.014. Epub 2010 Aug 12. Exp Eye Res. 2011. PMID: 20708611 Review. - Mitochondrial dysfunction and glaucoma.
Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Kong GY, et al. J Glaucoma. 2009 Feb;18(2):93-100. doi: 10.1097/IJG.0b013e318181284f. J Glaucoma. 2009. PMID: 19225343 Review.
Cited by
- Reactive Oxygen Species-Mediated Damage of Retinal Neurons: Drug Development Targets for Therapies of Chronic Neurodegeneration of the Retina.
Rohowetz LJ, Kraus JG, Koulen P. Rohowetz LJ, et al. Int J Mol Sci. 2018 Oct 27;19(11):3362. doi: 10.3390/ijms19113362. Int J Mol Sci. 2018. PMID: 30373222 Free PMC article. Review. - Inducible nitric oxide synthase-mediated alteration of mitochondrial OPA1 expression in ocular hypertensive rats.
Dai Y, Weinreb RN, Kim KY, Nguyen D, Park S, Sun X, Lindsey JD, Ellisman MH, Ju WK. Dai Y, et al. Invest Ophthalmol Vis Sci. 2011 Apr 16;52(5):2468-76. doi: 10.1167/iovs.10-5873. Invest Ophthalmol Vis Sci. 2011. PMID: 21220562 Free PMC article. - Mechanical environment of the optic nerve head in glaucoma.
Downs JC, Roberts MD, Burgoyne CF. Downs JC, et al. Optom Vis Sci. 2008 Jun;85(6):425-35. doi: 10.1097/OPX.0b013e31817841cb. Optom Vis Sci. 2008. PMID: 18521012 Free PMC article. Review. - Systemic Risk Factors in Branch Retinal Vein Occlusion: a Comprehensive Review.
Garnavou-Xirou C, Bontzos G, Smoustopoulos G, Velissaris S, Papadopoulos A, Georgopoulos E, Stavrakas P, Georgakopoulos C, Xirou T, Kozobolis V. Garnavou-Xirou C, et al. Maedica (Bucur). 2024 Jun;19(2):380-387. doi: 10.26574/maedica.2024.19.2.380. Maedica (Bucur). 2024. PMID: 39188832 Free PMC article. Review.
References
- Ahmed N. Advanced glycation end products—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. - PubMed
- Thornalley PJ. Cell activation by glycated proteins: AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand) 1998;44:1013–1023. - PubMed
- Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. - PubMed
- Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. - PubMed
- Ma L, Nicholson LF. Expression of the receptor for advanced glycation end products in Huntington’s disease caudate nucleus. Brain Res. 2004;1018:10–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical