Rer1p competes with APH-1 for binding to nicastrin and regulates gamma-secretase complex assembly in the early secretory pathway - PubMed (original) (raw)
Rer1p competes with APH-1 for binding to nicastrin and regulates gamma-secretase complex assembly in the early secretory pathway
Dragana Spasic et al. J Cell Biol. 2007.
Abstract
The gamma-secretase complex, consisting of presenilin, nicastrin, presenilin enhancer-2 (PEN-2), and anterior pharynx defective-1 (APH-1) cleaves type I integral membrane proteins like amyloid precursor protein and Notch in a process of regulated intramembrane proteolysis. The regulatory mechanisms governing the multistep assembly of this "proteasome of the membrane" are unknown. We characterize a new interaction partner of nicastrin, the retrieval receptor Rer1p. Rer1p binds preferentially immature nicastrin via polar residues within its transmembrane domain that are also critical for interaction with APH-1. Absence of APH-1 substantially increased binding of nicastrin to Rer1p, demonstrating the competitive nature of these interactions. Moreover, Rer1p expression levels control the formation of gamma-secretase subcomplexes and, concomitantly, total cellular gamma-secretase activity. We identify Rer1p as a novel limiting factor that negatively regulates gamma-secretase complex assembly by competing with APH-1 during active recycling between the endoplasmic reticulum (ER) and Golgi. We conclude that total cellular gamma-secretase activity is restrained by a secondary ER control system that provides a potential therapeutic value.
Figures
Figure 1.
Endogenous and overexpressed NCT and hRer1p coimmunoprecipitate. (A) mAb 9C3 and affinity-purified pAb B59.4 against NCT coimmunoprecipitate preferentially γ-secretase complex components (PS1-NTF, PEN-2, APH-1a, and PS1-CTF) from CHAPS- extracted HeLa cells (right lane; bound fraction), as this detergent preserves the integrity of the complex. Although hRer1p was also recovered in very small amounts, levels of coimmunoprecipitating hRer1p increased strongly when Triton X-100 was used for cell extraction (left lane; bound fraction). Other membrane proteins involved in ER-Golgi trafficking, like KDELr and BAP31, were not pulled down by NCT antibodies. Immunoprecipitations in the absence of extract, primary antibodies, or both were used as additional controls. (B–D) pAb against Rer1p coimmunoprecipitates small amounts of immature NCT from HeLa (B) and MEF (C) Triton X-100 extracts. DTBP cross-linking (0.75, 1.5, and 3 mM) before extraction slightly increased the coimmunoprecipitated immature NCT. In CHO that bears higher levels of immature NCT, the selective interaction with immature NCT was even more obvious (D). No PEN-2 (or other components; not depicted) coimmunoprecipitated with Rer1p. (E) Endogenous hRer1p interacts with overexpressed NCT but not PEN-2. Immunoprecipitation was performed with anti-hRer1p antibody upon transient overexpression of mouse NCT or PEN-2 in HeLa cells. When DTBP cross-linker was applied at two different concentrations (0.5 and 3 mM) before coimmunoprecipitation (right), the amount of NCT bound to hRer1p was increasing in a dose-dependent manner. Transiently overexpressed PEN-2 protein was used as a control and showed no interaction with hRer1p. When co-overexpressed, only NCT, not PEN-2, coimmunoprecipitated with anti-Rer1p (rightmost panel), again underscoring the specificity of the interaction. Black lines (D and E) indicate that total/unbound and bound lanes are from the same experiment/blot but different exposure times.
Figure 2.
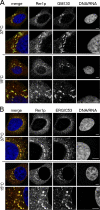
Endogenous hRer1p resides mainly in the IC. Fixed HeLa cells were processed for indirect double immunofluorescence labeling using antibodies against hRer1p and GM130 (A) or ERGIC-53 (B). Detection was done with Alexa 488 and 568 conjugated secondary antibodies followed by confocal laser-scanning microscopy. Immunostaining for GM130, a cis-Golgi matrix protein, and hRer1p were intimately but not identically distributed. Almost a complete overlap was found with anti–ERGIC-53, which marks the vesiculotubular elements of the IC. Colocalization was even more obvious when cells were preincubated at 15°C for 3 h (a condition that blocks transport from the IC; Saraste and Svensson, 1991) before fixation (A and B, bottom). Bars, 5 μm.
Figure 3.
hRer1p interacts with NCT through its TMD. (A) Schematic overview of the different NCT deletion constructs as well as chimeric TLN and NCT with swapped TMD. (B–D) Western blot analysis of coimmunoprecipitations with anti-hRer1p pAb from Triton X-100 extracts of HeLa cells transiently co-overexpressing hRer1p and different NCT deletion constructs. As a control, incubations without extract or extracts from mock-transfected cells were used. Total inputs are compared with bound fractions. Deletion of the IC slightly decreases binding, whereas ablation of IC and TMD almost completely abrogated interaction (B). Additional deletion of a short hydrophobic stretch upstream of the TMD abolished interaction completely (C). The interaction of hRer1p with a construct lacking the coding region for the entire ectodomain (NCTΔEC) demonstrates that the ectodomain is dispensable for the interaction with hRer1p. (E) Densitometric scanning and semiquantification of the hRer1p binding efficiencies toward the different NCT constructs, normalized to the binding of FL-NCT. Notice that NCTΔEC displays increased binding compared with intact NCT (mean ± SEM of three to four independent experiments). (F) The NCT TMD is sufficient for binding to hRer1p. Co-overexpression of the chimeric TLN construct, bearing only the TMD of NCT, with hRer1p followed by coimmunoprecipitation. TLN/TMDNCT but not FL-TLN coimmunoprecipitates with hRer1p using anti-hRer1p pAb. (G) Reciprocal experiment using chimeric NCT/TMDTLN (NCT bearing the TMD of TLN) co-overexpressed with hRer1p. Coimmunoprecipitation using anti-hRer1p pAb shows a greatly diminished interaction between NCT/TMDTLN and hRer1p compared with FL-NCT (semiquantified in E).
Figure 4.
Polar residues in the NCT TMD are critical for the interaction with Rer1p. (A, left) α-Helical wheel projection (using DNASTAR) of the TMD of NCT and Sec71p reveals a similar distribution of polar amino acid residues on one side of the helix (orange residues). These residues in Sec71p (but also Sec12p) have been shown to be critical for Rer1p binding. (right) Amino acid sequence of the TMDs of Rer1p interacting proteins as well as those of PEN-2, secretases, and TLN. The critical spacing of polar residues is only found in Rer1p interacting proteins (red boxed residues) and is not present in other TMDs (yellow boxed residues). The single Gly (green box) in NCT has been related to the binding of Aph-1 (Capell et al., 2003). (B) Sequence alignment of the extended TMD of NCT from different species (black/gray indicates identical/conserved residues). The indicated conserved polar amino acid residues, including Gly and Tyr within the NCT TMD (T670, G674, S681, T685, and Y686), are mutated to leucine. (C) Co-overexpression of FL-NCT and NCT bearing one or more point mutations within the TMD with hRer1p in HeLa cells, followed by coimmunoprecipitation using anti-hRer1p pAb. FL-NCT and NCT/TMDTLN are used as positive and negative control, respectively. Mutating individual polar residues extensively interferes with binding to hRer1p. Changing four or all indicated residues brings binding levels down to those of the chimeric NCT/TLNTMD. (D) Densitometric scanning and semiquantification of the Western blots from C. hRer1p binding for the different mutants is normalized to FL-NCT.
Figure 5.
Rer1p competes with APH-1 for binding to NCT. Triton X-100 extracts of WT MEFs and MEFs deficient in PS1 and -2, APH-1a, APH-1a,b,c, and NCT were used for coimmunoprecipitation experiments with mAb 9C3 against NCT to test whether Rer1p interacts with NCT in the absence of other γ-secretase complex components. In WT and PS1 and -2 KO MEFs, equal levels of endogenous Rer1p coimmunoprecipitated, and these levels clearly increased in APH-1a single and even more in APH-1a,b,c KO MEFs (arrow). As expected, no binding is observed in NCT−/− MEFs. This suggests that APH-1 isoforms compete with Rer1p for binding to NCT. The asterisk indicates a nonspecific band.
Figure 6.
Rer1p functions in NCT recycling between ER and Golgi. (A) hRer1p down-regulation affects NCT glycosylation. Western blot analysis of extracts from control and RNAi-treated HeLa cells pretreated without (NT) or with endoH (H) before SDS-PAGE. Knock down of hRer1p by >80% revealed an increased mobility of endoH-resistant (mature glycosylated) NCT (48 and 72 h, RNAi, H lane). (B) Representative metabolic pulse-chase experiment showing that Rer1p knockdown results in a higher mobility of the mature endoH-resistant NCT (arrowhead). Quantification of the endoH-resistant/total NCT over time (mean ± SEM) shows that the maturation of newly synthetized NCT is considerably delayed upon Rer1p knockdown, suggesting a longer residence time in early biosynthetic compartments. (C) NCT/TMDTLN is retained in the ER. Western blot of MEF WT, NCT−/− stably transduced with NCT, or NCT/TMDTLN (Fig. 3 A). In contrast to NCT, NCT/TMDTLN remains fully immature and fails to rescue PS1 endoproteolysis, PEN-2 and APH-1 stability, and APP-CTF processing. BN-PAGE clearly demonstrates that the TMD of NCT is required for interaction and γ-secretase complex formation. The black line indicates that the NCT−/− lanes were run on remote slots of the same gel. (D) The NCT TMD mediates an Rer1p-dependent ER-Golgi retrieval when introduced in a TLN reporter. TLN/TMDNCT, when stably transduced in NCT−/− MEF, presents with a sharp decrease in the levels of mature glycosylated chimeric TLN as compared with WT TLN. Subsequent down-regulation of endogenous Rer1p rescues mature glycosylation of TLN/TMDNCT (indicated by asterisk), demonstrating that Rer1p mediates its recycling/retrieval in early compartments through TMD interactions. (E) Western blot analysis of cell surface biotinylated proteins after 48 h of hRer1p knockdown. Down-regulation of hRer1p substantially increased the cell surface levels of mature NCT, PS1-NTF, and APH-1 in comparison to control treated cells, whereas transferrin receptor (Tfr) levels were unaffected. Equal inputs were used for all conditions, as shown by the total extracts.
Figure 7.
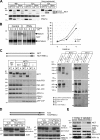
Changing the expression levels of Rer1p affects γ-secretase complex assembly and activity. (A and B) MEF WT, PS1 and -2 KO, and HeLa cells with normal and down-regulated Rer1p (48 h) and HeLa cells with transiently overexpressed hRer1p (36 h) were extracted in 0.5% dodecylmaltoside and processed for BN-PAGE. Control cells were transfected with empty vector (mock) or nonspecific duplex oligonucleotides (control). Western blot analysis for the different γ-secretase components indicates that down-regulation results in relatively increased levels of mature complexes (indicated by black box) in both HeLa (A; RNAi vs. control lane) and MEF WT cells (B). In contrast, overexpression of Rer1p in HeLa cells resulted in relatively less of the mature complex and more of the intermediate complexes. Suppression of hRer1p in MEF PS1 and -2 KO cells affects the stability of the NCT–APH-1a subcomplex (A). Identification of different dissociated complexes was done according to Fraering et al. (2004). Routinely, extract samples were tested on SDS-PAGE followed by Western blotting to verify the efficiency of Rer1p overexpression or down-regulation in HeLa and MEFs (bottom left). (C and D) hRer1p levels inversely correlate with cellular γ-secretase activity. (C) After 24 h of overexpression or 48 h of down-regulation (RNAi, specific duplex; NS, nonspecific control) of hRer1p in combination with overexpression of APP-C99, HeLa cells were metabolically labeled for 4 h as described previously (Annaert et al., 1999). Total secreted Aβ and APP-C99 were, respectively, immunoprecipitated from media and extracts and quantified by phosphorimaging. The ratio of Aβ to APP-C99 is significantly decreased or increased when hRer1p levels are up- or down-regulated (mean ± SEM; n = 5; t test *, P < 0.03; **, P < 0.05). (D) Cell-free γ-secretase assay. Extracts from control and hRer1p knockdown HeLa cells were mixed with affinity-purified recombinant APP-C99-FLAG (from transfected γ-secretase–deficient MEFs) and incubated at 37°C. Newly produced AICD-FLAG is clearly increased after hRer1p knockdown, indicating enhanced levels of γ-secretase activity.
Similar articles
- Immature nicastrin stabilizes APH-1 independent of PEN-2 and presenilin: identification of nicastrin mutants that selectively interact with APH-1.
Shirotani K, Edbauer D, Kostka M, Steiner H, Haass C. Shirotani K, et al. J Neurochem. 2004 Jun;89(6):1520-7. doi: 10.1111/j.1471-4159.2004.02447.x. J Neurochem. 2004. PMID: 15189355 - The presenilin C-terminus is required for ER-retention, nicastrin-binding and gamma-secretase activity.
Kaether C, Capell A, Edbauer D, Winkler E, Novak B, Steiner H, Haass C. Kaether C, et al. EMBO J. 2004 Dec 8;23(24):4738-48. doi: 10.1038/sj.emboj.7600478. Epub 2004 Nov 18. EMBO J. 2004. PMID: 15549135 Free PMC article. - Aph-1 interacts at the cell surface with proteins in the active gamma-secretase complex and membrane-tethered Notch.
Hansson EM, Strömberg K, Bergstedt S, Yu G, Näslund J, Lundkvist J, Lendahl U. Hansson EM, et al. J Neurochem. 2005 Mar;92(5):1010-20. doi: 10.1111/j.1471-4159.2004.02926.x. J Neurochem. 2005. PMID: 15715652 - Building gamma-secretase: the bits and pieces.
Spasic D, Annaert W. Spasic D, et al. J Cell Sci. 2008 Feb 15;121(Pt 4):413-20. doi: 10.1242/jcs.015255. J Cell Sci. 2008. PMID: 18256384 Review. - Functional reconstitution of gamma-secretase through coordinated expression of presenilin, nicastrin, Aph-1, and Pen-2.
Periz G, Fortini ME. Periz G, et al. J Neurosci Res. 2004 Aug 1;77(3):309-22. doi: 10.1002/jnr.20203. J Neurosci Res. 2004. PMID: 15248287 Review.
Cited by
- High temperature promotes amyloid β-protein production and γ-secretase complex formation via Hsp90.
Noorani AA, Yamashita H, Gao Y, Islam S, Sun Y, Nakamura T, Enomoto H, Zou K, Michikawa M. Noorani AA, et al. J Biol Chem. 2020 Dec 25;295(52):18010-18022. doi: 10.1074/jbc.RA120.013845. Epub 2020 Oct 16. J Biol Chem. 2020. PMID: 33067321 Free PMC article. - Presenilin 1 and Presenilin 2 Target γ-Secretase Complexes to Distinct Cellular Compartments.
Meckler X, Checler F. Meckler X, et al. J Biol Chem. 2016 Jun 10;291(24):12821-12837. doi: 10.1074/jbc.M115.708297. Epub 2016 Apr 8. J Biol Chem. 2016. PMID: 27059953 Free PMC article. - G206D Mutation of Presenilin-1 Reduces Pen2 Interaction, Increases Aβ42/Aβ40 Ratio and Elevates ER Ca(2+) Accumulation.
Chen WT, Hsieh YF, Huang YJ, Lin CC, Lin YT, Liu YC, Lien CC, Cheng IH. Chen WT, et al. Mol Neurobiol. 2015 Dec;52(3):1835-1849. doi: 10.1007/s12035-014-8969-1. Epub 2014 Nov 15. Mol Neurobiol. 2015. PMID: 25394380 - GABAB receptor cell-surface export is controlled by an endoplasmic reticulum gatekeeper.
Doly S, Shirvani H, Gäta G, Meye FJ, Emerit MB, Enslen H, Achour L, Pardo-Lopez L, Yang SK, Armand V, Gardette R, Giros B, Gassmann M, Bettler B, Mameli M, Darmon M, Marullo S. Doly S, et al. Mol Psychiatry. 2016 Apr;21(4):480-90. doi: 10.1038/mp.2015.72. Epub 2015 Jun 2. Mol Psychiatry. 2016. PMID: 26033241 Free PMC article. - The ER retention protein RER1 promotes alpha-synuclein degradation via the proteasome.
Park HJ, Ryu D, Parmar M, Giasson BI, McFarland NR. Park HJ, et al. PLoS One. 2017 Sep 6;12(9):e0184262. doi: 10.1371/journal.pone.0184262. eCollection 2017. PLoS One. 2017. PMID: 28877262 Free PMC article.
References
- Annaert, W., and B. De Strooper. 1999. Presenilins: molecular switches between proteolysis and signal transduction. Trends Neurosci. 22:439–443. - PubMed
- Annaert, W., and B. De Strooper. 2002. A cell biological perspective on Alzheimer's disease. Annu. Rev. Cell Dev. Biol. 18:25–51. - PubMed
- Annaert, W.G., L. Levesque, K. Craessaerts, I. Dierinck, G. Snellings, D. Westaway, P. St. George-Hyslop, B. Cordell, P. Fraser, and B. De Strooper. 1999. Presenilin 1 controls γ-secretase processing of the amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J. Cell Biol. 147:277–294. - PMC - PubMed
- Annaert, W.G., C. Esselens, V. Baert, C. Boeve, G. Snellings, P. Cupers, K. Craessaerts, and B. De Strooper. 2001. Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron. 32:579–589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources