Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons - PubMed (original) (raw)
Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons
Ian R Wickersham et al. Neuron. 2007.
Abstract
There has never been a wholesale way of identifying neurons that are monosynaptically connected either to some other cell group or, especially, to a single cell. The best available tools, transsynaptic tracers, are unable to distinguish weak direct connections from strong indirect ones. Furthermore, no tracer has proven potent enough to label any connected neurons whatsoever when starting from a single cell. Here we present a transsynaptic tracer that crosses only one synaptic step, unambiguously identifying cells directly presynaptic to the starting population. Based on rabies virus, it is genetically targetable, allows high-level expression of any gene of interest in the synaptically coupled neurons, and robustly labels connections made to single cells. This technology should enable a far more detailed understanding of neural connectivity than has previously been possible.
Figures
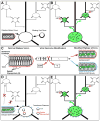
Figure 1. Transcomplemented Transsynaptic Tracing
(A) A deletion mutant tracing virus missing one or more genes required for transsynaptic spread, as well as the separate missing viral gene(s), are introduced into a cell or cell type of interest. Both the initial infection and the complementing viral genes must be restricted to the neuronal population of interest. (B) Because all viral genes are present in the initially infected population, the virus can spread transsynaptically to cells in direct synaptic contact with them. Since the missing viral genes are not present in these cells, however, the virus cannot spread further. (C) The rabies virion. The viral core consists of the RNA genome and associated proteins and is surrounded by a host-cell-derived membrane in which is embedded the rabies virus glycoprotein (G). The EGFP gene was substituted for that of the glycoprotein within the viral genome (center). Versions of this virus can be made that incorporate either its native glycoprotein (designated SADΔG-EGFP, right, top) or the glycoprotein of some other virus. In this study the rabies virus was pseudotyped with the glycoprotein from ASLV-A, termed EnvA, and this virus is designated SADΔG-EGFP(EnvA) (right, bottom). (D) Targeting infection. ASLV-A-pseudotyped rabies virus [SADΔG-EGFP(EnvA)] can’t infect mammalian neurons unless the gene for ASLV-A’s receptor, TVA, has been introduced into them. This causes the receptor to be expressed on the cells’ surface, allowing infection by the pseudotyped virus. Therefore, the basic requirements of the system are as follows. First insert two genes into the cell or cell type of interest: the gene for TVA, so the virus can enter, and the gene for the rabies virus glycoprotein, so the virus can spread to synaptically coupled cells. Then apply the ASLV-A-pseudotyped virus. (E) Following these steps, there is specific infection of the TVA-expressing cell; complementation with the rabies virus glycoprotein allows the virus to spread to directly presynaptic neurons. These cells all express the EGFP encoded in the viral genome, but the virus cannot spread beyond these directly connected cells because they do not express the viral glycoprotein.
Figure 2. Selective Infection and In Situ Complementation in Slice Culture
(A–D) Initial infection is restricted to cells expressing the ASLV-A receptor, TVA. In these control experiments to test infection selectivity, isolated neurons in cultured brain slices were transfected using the gene gun with two genes, one encoding TVA, and the other, DsRed2. ASLV-A-pseudotyped rabies virus [SADΔG-EGFP(EnvA)] was applied the next day and images were taken 6 days following. (A and C) DsRed2 expression marking transfection with TVA and (B and D) EGFP expression indicating subsequent selective infection of the same TVA-expressing cells with pseudotyped virus. (E–H) In situ complementation permits transsynaptic spread from a single initially infected cell to a cluster of monosynaptically connected cells. Isolated neurons were transfected with three genes encoding TVA to permit viral infection, DsRed2 to mark transfected cells, and the rabies virus glycoprotein gene to complement the deletion in the viral genome. (E) DsRed2 fluorescence indicating a transfected cell, marked with a dotted line, at the center of the cluster shown in (F). (G and H) Two more examples of clusters surrounding a single transfected cell. The initially infected cells expressing both EGFP and DsRed2 appear yellow. Scale bars: 200 μm.
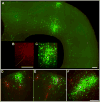
Figure 3. More Examples of Transcomplemented Tracing
(A–C) Long-range viral spread from a single initially infected cell. (A) A huge cluster of green cells surrounds a single red/green deep-layer cortical neuron (dotted line) at 8 days postinfection. Another dense cluster of cells is also infected in the superficial cortical layers immediately above it, consistent with known projections of superficial layers to deeper ones; distant deep-layer pyramidal cells are also infected, again consistent with known patterns of long-range intralaminar connectivity. To the left of the putatively initially infected cell is a second yellow (double-labeled) cell, apparently secondarily—and recently—infected because of the lack of green cells surrounding it. (B–C) Closeup of central cluster from (A). (D–F) More examples of in situ complementation: clusters of infected cells surrounding isolated putatively postsynaptic ones. Scale bars: 200 μm.
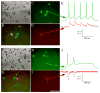
Figure 4. Viral Spread Is Specific to Cells Presynaptic to the Initially Infected Cell
(A) DIC image of slice and recording pipettes targeting putatively pre- and postsynaptic neurons, (B) combined fluorescent image, and (C–D) single-channel fluorescence images. (E) Inhibitory postsynaptic currents in the putatively postsynaptic cell are coincident with action potentials in a nearby infected one, demonstrating a monosynaptic connection. (F–J) Similar demonstration of spread to an excitatory pre-synaptic cell. Scale bar: 100 μm, applies to all panels.
Similar articles
- Rabies virus-based barcoded neuroanatomy resolved by single-cell RNA and in situ sequencing.
Zhang A, Jin L, Yao S, Matsuyama M, van Velthoven CTJ, Sullivan HA, Sun N, Kellis M, Tasic B, Wickersham I, Chen X. Zhang A, et al. Elife. 2024 Feb 6;12:RP87866. doi: 10.7554/eLife.87866. Elife. 2024. PMID: 38319699 Free PMC article. - Tracing synaptic connectivity onto embryonic stem cell-derived neurons.
Garcia I, Huang L, Ung K, Arenkiel BR. Garcia I, et al. Stem Cells. 2012 Oct;30(10):2140-51. doi: 10.1002/stem.1185. Stem Cells. 2012. PMID: 22996827 Free PMC article. - Transgenic targeting of recombinant rabies virus reveals monosynaptic connectivity of specific neurons.
Weible AP, Schwarcz L, Wickersham IR, Deblander L, Wu H, Callaway EM, Seung HS, Kentros CG. Weible AP, et al. J Neurosci. 2010 Dec 8;30(49):16509-13. doi: 10.1523/JNEUROSCI.2442-10.2010. J Neurosci. 2010. PMID: 21147990 Free PMC article. - G gene-deficient single-round rabies viruses for neuronal circuit analysis.
Ghanem A, Conzelmann KK. Ghanem A, et al. Virus Res. 2016 May 2;216:41-54. doi: 10.1016/j.virusres.2015.05.023. Epub 2015 Jun 8. Virus Res. 2016. PMID: 26065596 Review. - The role of neural activity in cortical axon branching.
Uesaka N, Ruthazer ES, Yamamoto N. Uesaka N, et al. Neuroscientist. 2006 Apr;12(2):102-6. doi: 10.1177/1073858405281673. Neuroscientist. 2006. PMID: 16514007 Review.
Cited by
- The synaptic inputs and thalamic projections of two classes of layer 6 corticothalamic neurons in primary somatosensory cortex of the mouse.
Whilden CM, Chevée M, An SY, Brown SP. Whilden CM, et al. J Comp Neurol. 2021 Dec;529(17):3751-3771. doi: 10.1002/cne.25163. Epub 2021 May 6. J Comp Neurol. 2021. PMID: 33908623 Free PMC article. - Vesicular stomatitis virus with the rabies virus glycoprotein directs retrograde transsynaptic transport among neurons in vivo.
Beier KT, Saunders AB, Oldenburg IA, Sabatini BL, Cepko CL. Beier KT, et al. Front Neural Circuits. 2013 Feb 7;7:11. doi: 10.3389/fncir.2013.00011. eCollection 2013. Front Neural Circuits. 2013. PMID: 23403489 Free PMC article. - Long-range GABAergic projections contribute to cortical feedback control of sensory processing.
Mazo C, Nissant A, Saha S, Peroni E, Lledo PM, Lepousez G. Mazo C, et al. Nat Commun. 2022 Nov 12;13(1):6879. doi: 10.1038/s41467-022-34513-0. Nat Commun. 2022. PMID: 36371430 Free PMC article. - Development and plasticity of the primary visual cortex.
Espinosa JS, Stryker MP. Espinosa JS, et al. Neuron. 2012 Jul 26;75(2):230-49. doi: 10.1016/j.neuron.2012.06.009. Neuron. 2012. PMID: 22841309 Free PMC article. Review. - Fluorescent transgenic mouse models for whole-brain imaging in health and disease.
Arias A, Manubens-Gil L, Dierssen M. Arias A, et al. Front Mol Neurosci. 2022 Sep 23;15:958222. doi: 10.3389/fnmol.2022.958222. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36211979 Free PMC article. Review.
References
- Barnard RJ, Elleder D, Young JA. Avian sarcoma and leukosis virus-receptor interactions: from classical genetics to novel insights into virus-cell membrane fusion. Virology. 2006;344:25–29. - PubMed
- Bates P, Young JA, Varmus HE. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. - PubMed
- Borrell V, Yoshimura Y, Callaway EM. Targeted gene delivery to telencephalic inhibitory neurons by directional in utero electroporation. J Neurosci Methods. 2005;143:151–158. - PubMed
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY010742-16/EY/NEI NIH HHS/United States
- MH63912/MH/NIMH NIH HHS/United States
- R01 EY010742/EY/NEI NIH HHS/United States
- R01 MH063912/MH/NIMH NIH HHS/United States
- CA70810/CA/NCI NIH HHS/United States
- EY10742/EY/NEI NIH HHS/United States
- R01 CA070810/CA/NCI NIH HHS/United States
- R01 MH063912-08/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials