Perturbation of endoplasmic reticulum homeostasis facilitates prion replication - PubMed (original) (raw)
Comparative Study
. 2007 Apr 27;282(17):12725-33.
doi: 10.1074/jbc.M611909200. Epub 2007 Feb 28.
Affiliations
- PMID: 17329244
- PMCID: PMC2804266
- DOI: 10.1074/jbc.M611909200
Comparative Study
Perturbation of endoplasmic reticulum homeostasis facilitates prion replication
Claudio Hetz et al. J Biol Chem. 2007.
Abstract
Prion diseases are fatal and infectious neurodegenerative disorders characterized by the accumulation of an abnormally folded form of the prion protein (PrP), termed PrP(Sc). Prion replication triggers endoplasmic reticulum (ER) stress, neuronal dysfunction, and apoptosis. In this study we analyze the effect of perturbations in ER homeostasis on PrP biochemical properties and prion replication. ER stress led to the generation of a mis-folded PrP isoform, which is detergent-insoluble and protease-sensitive. To understand the mechanism by which ER stress generates PrP misfolding, we assessed the contribution of different signaling pathways implicated in the unfolded protein response. Expression of a dominant negative form of IRE1 alpha or XBP-1 significantly increased PrP aggregation, whereas overexpression of ATF4 or an active mutant form of XBP-1 and ATF6 had the opposite affect. Analysis of prion replication in vitro revealed that the PrP isoform generated after ER stress is more efficiently converted into PrP(Sc) compared with the protein extracted from untreated cells. These findings indicate that ER-damaged cells might be more susceptible to prion replication. Because PrP(Sc) induces ER stress, our data point to a vicious cycle accelerating prion replication, which may explain the rapid progression of the disease.
Figures
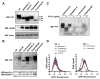
FIGURE 1. Proteasome inhibitors induce ER stress in Neuro2A cells
A, N2A-mPrP cells were treated with 15 _μ_M MG132 (MG) or 6 _μ_M epoxomycin (Epox) for 16 h, and the levels of PrP, GADD153/CHOP, Grp58, Grp78/Bip, and pro-caspase-12 were determined by Western blot. B, Neuro2A cells stably transfected with a dominant negative form of caspase-12 (caspase-12DN) or empty vector (mock) were treated with 6 _μ_M epoxomycin (Epox), 15 _μ_M lactacystin (Lacta), or 15 _μ_M MG132, and after 24 h of incubation cell viability was determined by the MTS assay. Data show the mean ± S.D. of two different experiments performed in triplicate. NT, non-treated cells.
FIGURE 2. ER stress alters the physicochemical properties of PrP independent of the proteasome
N2A-mPrP cells were treated for 16 h with 6 _μ_M epoxomycin, 50 _μ_M brefeldin A, 1.3 _μ_M A23187, 1 _μ_g/ml tunicamycin, or 6 _μ_M thapsigargin. A, in cells undergoing ER stress, total PrP, GADD153/CHOP, and actin levels were determined by Western blot. In the upper panel, D-, M-, and N- correspond to the di-, mono-, and non-glycosylated forms of PrP. B, in parallel, after the indicated treatments, detergent insolubility was determined in samples centrifuged in 5% Sarkosyl, and the quantity of PrP in the pellet was analyzed by Western blot. Co-precipitation of Grp58 and Grp78 in the same samples is shown. C, protein extracts from cells treated with ER stressors or epoxomycin were digested with 5 _μ_g/ml PK or left untreated, and PrP levels were analyzed by Western blot. D, proteasome activity was monitored in cells stably expressing GFPu by fluorescence-activated cell sorting analysis. GFP fluorescence emission was determined in cells treated for 6 h with ER stress inducers (50 _μ_M brefeldin A, 6 _μ_M thapsigargin, 1 _μ_g/ml tunicamycin, or 1.3 _μ_M A23187) or proteasome inhibitors (6 _μ_M epoxomycin or 15 _μ_M lactacystin), and data are shown in the right and left graphs, respectively. NT, non-treated cells.
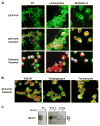
FIGURE 3. Subcellular localization of PrP in cells undergoing ER stress
A, Neuro2A cells expressing a GFP-PrP fusion protein were treated with 15 _μ_M lactacystin or 50 _μ_M brefeldin A, and PrP distribution was analyzed by immunofluorescence (green fluorescence). As intracellular markers, calnexin staining (red fluorescence) and nuclear staining (Hoechst, blue) were used. Superposition of PrP and calnexin staining resulted in a yellow color. B, merged pictures from GFP-PrP and calnexin staining is shown in cells treated with 6 _μ_M thapsigargin, 1 _μ_g/ml tunicamycin, or 1.3 _μ_M A23187 for 16 h. C, cells treated with tunicamycin (Tunica.) or brefeldin A (Bref. A) as described in Fig. 3B, were treated with phosphatidylinositol-phospholipase C (PI-PLC) for 5 h or left untreated, and detergent-insoluble PrP was separated as described under “Materials and Methods.” NT, non-treated cells.
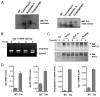
FIGURE 4. ER stress triggers the aggregation of PrPC in primary neuronal cultures
A, primary cortical neurons from embryonic day E16.5 were treated with 10 _μ_g/ml tunicamycin, 1 _μ_M thapsigargin, or 20 _μ_M brefeldin A for 16 h. The level of detergent-insoluble PrP was determined in protein extracts centrifuged in 5% Sarkosyl by measuring the quantity of PrP in the pellet by Western blot (left blot). Total levels of PrP are shown in the same extracts (right blot). B, primary cortical neurons were treated with 10 _μ_g/ml tunicamycin for the indicated time points, and the splicing of xbp-1 mRNA was determined by reverse transcription-PCR of total cDNA samples. Spliced and non-spliced XBP-1 PCR fragments are indicated. C, in parallel, primary neuronal cultures were treated with 10 _μ_g/ml tunicamycin, 1 _μ_M thapsigargin, or 20 _μ_M brefeldin A for the indicated time points or left untreated (NT), and the expression levels of spliced XBP-1 protein (XBP-1s) and ATF4 were analyzed by Western blot. D, up-regulation of the mRNA for grp78/BiP, grp58, chop/gadd153, and xbp-1 was quantified by real-time PCR and normalized with the levels of _β_-actin in cells treated with 10 _μ_g/ml tunicamycin (Tm) for 8 h.
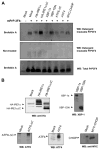
FIGURE 5. Activation of the UPR decreases PrPC aggregation under ER stress conditions
A, Neuro2A cells were transiently co-transfected with an expression vector for mPrP-3F4 in the presence or absence of expression vectors for HA-IRE1_α_, HA-IRE1_α_ΔC (dominant negative form of IRE1_α_), XBP-1 active form (XBP-1s, spliced XBP-1), dominant negative XBP-1 (XBP-1DN), active ATF6 (ATF6ΔC), ATF4, MYC-CHOP, or empty pDNA.3 vector (mock). Forty-eight hours after the transfection, cells were treated with 10 μ_M brefeldin A for 16 h or left untreated, and the generation of detergent-insoluble PrP-3F4 species was analyzed by Western blot. All results are representative of three independent experiments. B, control experiments to assess the expression levels by Western blot analysis of HA-IRE1_α, HA-IRE1_α_ΔC, XBP-1s, XBP-1DN, ATF6ΔC, ATF4, and MYC-CHOP in the experiments described in A.
FIGURE 6. ER stress facilitates prion replication
A, schematic representation of the experimental procedure used to study the influence of ER stress on prion replication. B, Neuro2A cells stably expressing mPrP-3F4 were treated with 50 _μ_M brefeldin A (Bref. A) or 6 _μ_M epoxomycin (Epox) for 16 h, and post-nuclear cell lysates were mixed with Rocky Mountain Laboratory scrapie brain homogenate (at 1:100 or 1:300 dilution) and subjected to eight cycles of incubation and sonication (PMCA). The formation of protease-resistance PrPSc was analyzed by Western blot after treatment of extracts with 50 _μ_g/ml of PK as described under “Materials and Methods.” The levels of PrP-3F4 in the total extracts before PK treatment are shown in the right panel.
FIGURE 7. Schematic model for the relationship between alterations in ER homeostasis, PrP misfolding, and neurodegeneration
Our findings suggest that ER stress may play a central role in prion replication and neurodegeneration associated with TSEs. The data points to a vicious cycle in which PrPSc formation promotes ER stress, which in turn facilitates prion replication by inducing the partial misfolding and aggregation of PrPC (denoted as PrP*). Activation of the UPR by ER stress conditions may have a protective effect by preventing the misfolding of PrP.
Similar articles
- Stressing out the ER: a role of the unfolded protein response in prion-related disorders.
Hetz CA, Soto C. Hetz CA, et al. Curr Mol Med. 2006 Feb;6(1):37-43. doi: 10.2174/156652406775574578. Curr Mol Med. 2006. PMID: 16472111 Free PMC article. Review. - Molecular mechanisms of neurotoxicity of pathological prion protein.
Castilla J, Hetz C, Soto C. Castilla J, et al. Curr Mol Med. 2004 Jun;4(4):397-403. doi: 10.2174/1566524043360654. Curr Mol Med. 2004. PMID: 15354870 Review. - The retention of prion protein in the endoplasmic reticulum prevents N2A cells from proteasome inhibition-induced cytotoxicity.
Fang S, Wang R, Liu H, Zhuang W, Wang Z, Zhang J, Pei L, Liu Y, Su Y. Fang S, et al. Biochem Biophys Res Commun. 2017 Sep 16;491(2):500-507. doi: 10.1016/j.bbrc.2017.06.176. Epub 2017 Jun 29. Biochem Biophys Res Commun. 2017. PMID: 28669732 - Trapping prion protein in the endoplasmic reticulum impairs PrPC maturation and prevents PrPSc accumulation.
Cardinale A, Filesi I, Vetrugno V, Pocchiari M, Sy MS, Biocca S. Cardinale A, et al. J Biol Chem. 2005 Jan 7;280(1):685-94. doi: 10.1074/jbc.M407360200. Epub 2004 Oct 28. J Biol Chem. 2005. PMID: 15513919 - Prion Infectivity Plateaus and Conversion to Symptomatic Disease Originate from Falling Precursor Levels and Increased Levels of Oligomeric PrPSc Species.
Mays CE, van der Merwe J, Kim C, Haldiman T, McKenzie D, Safar JG, Westaway D. Mays CE, et al. J Virol. 2015 Dec;89(24):12418-26. doi: 10.1128/JVI.02142-15. Epub 2015 Sep 30. J Virol. 2015. PMID: 26423957 Free PMC article.
Cited by
- The Protein-disulfide Isomerase ERp57 Regulates the Steady-state Levels of the Prion Protein.
Torres M, Medinas DB, Matamala JM, Woehlbier U, Cornejo VH, Solda T, Andreu C, Rozas P, Matus S, Muñoz N, Vergara C, Cartier L, Soto C, Molinari M, Hetz C. Torres M, et al. J Biol Chem. 2015 Sep 25;290(39):23631-45. doi: 10.1074/jbc.M114.635565. Epub 2015 Jul 13. J Biol Chem. 2015. PMID: 26170458 Free PMC article. - Expression of mutant or cytosolic PrP in transgenic mice and cells is not associated with endoplasmic reticulum stress or proteasome dysfunction.
Quaglio E, Restelli E, Garofoli A, Dossena S, De Luigi A, Tagliavacca L, Imperiale D, Migheli A, Salmona M, Sitia R, Forloni G, Chiesa R. Quaglio E, et al. PLoS One. 2011 Apr 29;6(4):e19339. doi: 10.1371/journal.pone.0019339. PLoS One. 2011. PMID: 21559407 Free PMC article. - Proteasomal dysfunction and endoplasmic reticulum stress enhance trafficking of prion protein aggregates through the secretory pathway and increase accumulation of pathologic prion protein.
Nunziante M, Ackermann K, Dietrich K, Wolf H, Gädtke L, Gilch S, Vorberg I, Groschup M, Schätzl HM. Nunziante M, et al. J Biol Chem. 2011 Sep 30;286(39):33942-53. doi: 10.1074/jbc.M111.272617. Epub 2011 Aug 11. J Biol Chem. 2011. PMID: 21835918 Free PMC article. - Redox control of prion and disease pathogenesis.
Singh N, Singh A, Das D, Mohan ML. Singh N, et al. Antioxid Redox Signal. 2010 Jun 1;12(11):1271-94. doi: 10.1089/ars.2009.2628. Antioxid Redox Signal. 2010. PMID: 19803746 Free PMC article. Review. - Synaptic dysfunction in prion diseases: a trafficking problem?
Senatore A, Restelli E, Chiesa R. Senatore A, et al. Int J Cell Biol. 2013;2013:543803. doi: 10.1155/2013/543803. Epub 2013 Nov 28. Int J Cell Biol. 2013. PMID: 24369467 Free PMC article. Review.
References
- Hegde RS, Rane NS. Trends Neurosci. 2003;26:337–339. - PubMed
- Harris DA. Br Med Bull. 2003;66:71–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS050349/NS/NINDS NIH HHS/United States
- R01 NS050349-03/NS/NINDS NIH HHS/United States
- R01 NS050349/NS/NINDS NIH HHS/United States
- R01 NS049173/NS/NINDS NIH HHS/United States
- NS049173/NS/NINDS NIH HHS/United States
- R01 NS049173-04/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials