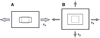Mechanoregulation of gene expression in fibroblasts - PubMed (original) (raw)
Review
Mechanoregulation of gene expression in fibroblasts
James H-C Wang et al. Gene. 2007.
Abstract
Mechanical loads placed on connective tissues alter gene expression in fibroblasts through mechanotransduction mechanisms by which cells convert mechanical signals into cellular biological events, such as gene expression of extracellular matrix components (e.g., collagen). This mechanical regulation of ECM gene expression affords maintenance of connective tissue homeostasis. However, mechanical loads can also interfere with homeostatic cellular gene expression and consequently cause the pathogenesis of connective tissue diseases such as tendinopathy and osteoarthritis. Therefore, the regulation of gene expression by mechanical loads is closely related to connective tissue physiology and pathology. This article reviews the effects of various mechanical loading conditions on gene regulation in fibroblasts and discusses several mechanotransduction mechanisms. Future research directions in mechanoregulation of gene expression are also suggested.
Figures
Fig. 1
An illustration of 2-D in vitro models for studying mechanobiological responses of fibroblasts. A. Uniaxial stretching. B. Biaxial stretching. When two stretches (ε_x_ and ε_y_) are equal, it is referred to as equibiaxial stretching.
Fig. 2
Shown is the fibroblast-populated collagen gel (FPCG). This type of 3-D in vitro model has been used to study the effects of internal mechanical gel tension on fibroblasts. Also, an external mechanical stretching can be applied to the gel in this model. Note that the fibroblasts in the gel generate tension, and as a result, the gel changes from rectangular (arrow) to parabolic shape (triangle).
Fig. 3
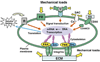
A conceptual illustration of several cellular mechanotransduction mechanisms. Mechanical loads can induce signal transduction by directly transmitting forces from the extracellular matrix (ECM) to integrins, the cytoskeleton, and the nucleus, eventually resulting in changes in gene transcription and protein translation. Also, mechanical stretching of cells opens stretching-activated channels (SACs), causing influx of ions (e.g., Ca+) and thus a series of downstream signaling events. Still, mechanical loads acting on a cell may unfold a domain of the extracellular protein (M) and expose a cryptic site that may serve as an activating ligand for a cell surface receptor, which results in a series of signaling events. Additionally, mechanical load applied to a “force receptor” (FR) may initiate signal transduction, which results in transcription, followed by translation. As a result, soluble factors are secreted into the ECM which act on the receptor (R) and then initiate a cascade of signaling events. Other signaling molecules that are involved in mechanotransduction can include CD44 transmembrane protein and its intracellular domain (CD44ICD), which is translocated into the nucleus causing gene transcription.
Similar articles
- Mechanotransduction and extracellular matrix homeostasis.
Humphrey JD, Dufresne ER, Schwartz MA. Humphrey JD, et al. Nat Rev Mol Cell Biol. 2014 Dec;15(12):802-12. doi: 10.1038/nrm3896. Epub 2014 Oct 22. Nat Rev Mol Cell Biol. 2014. PMID: 25355505 Free PMC article. Review. - Gene regulation by mechanotransduction in fibroblasts.
Chiquet M, Tunç-Civelek V, Sarasa-Renedo A. Chiquet M, et al. Appl Physiol Nutr Metab. 2007 Oct;32(5):967-73. doi: 10.1139/H07-053. Appl Physiol Nutr Metab. 2007. PMID: 18059623 Review. - Integrins and extracellular matrix in mechanotransduction.
Schwartz MA. Schwartz MA. Cold Spring Harb Perspect Biol. 2010 Dec;2(12):a005066. doi: 10.1101/cshperspect.a005066. Epub 2010 Nov 17. Cold Spring Harb Perspect Biol. 2010. PMID: 21084386 Free PMC article. Review. - From mechanotransduction to extracellular matrix gene expression in fibroblasts.
Chiquet M, Gelman L, Lutz R, Maier S. Chiquet M, et al. Biochim Biophys Acta. 2009 May;1793(5):911-20. doi: 10.1016/j.bbamcr.2009.01.012. Epub 2009 Jan 31. Biochim Biophys Acta. 2009. PMID: 19339214 Review. - How do fibroblasts translate mechanical signals into changes in extracellular matrix production?
Chiquet M, Renedo AS, Huber F, Flück M. Chiquet M, et al. Matrix Biol. 2003 Mar;22(1):73-80. doi: 10.1016/s0945-053x(03)00004-0. Matrix Biol. 2003. PMID: 12714044 Review.
Cited by
- Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases.
Popov C, Burggraf M, Kreja L, Ignatius A, Schieker M, Docheva D. Popov C, et al. BMC Mol Biol. 2015 Mar 13;16:6. doi: 10.1186/s12867-015-0036-6. BMC Mol Biol. 2015. PMID: 25880261 Free PMC article. - Establishment of SV40 Large T-Antigen-Immortalized Yak Rumen Fibroblast Cell Line and the Fibroblast Responses to Lipopolysaccharide.
Wang J, Yue Z, Che L, Li H, Hu R, Shi L, Zhang X, Zou H, Peng Q, Jiang Y, Wang Z. Wang J, et al. Toxins (Basel). 2023 Aug 31;15(9):537. doi: 10.3390/toxins15090537. Toxins (Basel). 2023. PMID: 37755963 Free PMC article. - Tissue specific human fibroblast differential expression based on RNAsequencing analysis.
Foote AG, Wang Z, Kendziorski C, Thibeault SL. Foote AG, et al. BMC Genomics. 2019 Apr 23;20(1):308. doi: 10.1186/s12864-019-5682-5. BMC Genomics. 2019. PMID: 31014251 Free PMC article. - Skin tissue regeneration for burn injury.
Shpichka A, Butnaru D, Bezrukov EA, Sukhanov RB, Atala A, Burdukovskii V, Zhang Y, Timashev P. Shpichka A, et al. Stem Cell Res Ther. 2019 Mar 15;10(1):94. doi: 10.1186/s13287-019-1203-3. Stem Cell Res Ther. 2019. PMID: 30876456 Free PMC article. Review. - Gene expression profile in pelvic organ prolapse.
Brizzolara SS, Killeen J, Urschitz J. Brizzolara SS, et al. Mol Hum Reprod. 2009 Jan;15(1):59-67. doi: 10.1093/molehr/gan074. Epub 2008 Dec 4. Mol Hum Reprod. 2009. PMID: 19056808 Free PMC article.
References
- Abrahams M. Mechanical behaviour of tendon in vitro. A preliminary report. Med. Biol. Eng. 1967;5:433–443. - PubMed
- Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Med. Sci. Sports Exerc. 1993;25:603–607. - PubMed
- Amano M, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. - PubMed
- Amano M, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
- Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J. Orthop. Res. 2002a;20:36–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources