Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis - PubMed (original) (raw)
Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis
Wei Yu et al. Mol Biol Cell. 2007 May.
Abstract
Many organs consist of a hollow cavity surrounded by a monolayer of epithelial cells. Despite their common structure, such organs form by diverse morphogenetic processes. Three-dimensional culture systems have been useful in analyzing the events. Most processes require a combination of cell proliferation and cell death to produce a hollow cavity. Here, we describe a new three-dimensional culture system in which primary human lung alveolar type II cells formed hollow epithelial cysts by a novel process. Individual cells moved, collided, and formed alveolar-like cysts without appreciable proliferation or apoptosis. The alveolar-like cysts consisted of a polarized monolayer of differentiated alveolar type II cells, which secreted surfactant into the central lumen. Blockage of beta1 integrin did not alter cell movement or collision, but it greatly reduced adhesion of cells after collision and subsequent formation of alveolar-like cysts. Treatment of preformed alveolar-like cysts with forskolin increased their diameter, possibly due to stimulation of fluid secretion into the lumen. We conclude that epithelial differentiation and cyst formation can occur without appreciable proliferation or apoptosis.
Figures
Figure 1.
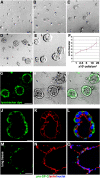
AT II cells form ALCs in Matrigel culture. (A–E) Phase-contrast pictures after 4 d in 2% Matrigel cultures; cells were initially plated at 1, 2.5, 5, 10, and 25 × 104 cells/cm2. (F) Final ALC size was dependent on initial cell density. (G–O) Evidence that ALCs were composed of AT II cells. (G) Confocal images show ALCs stained with LysoTracker green DND-26, a fluorescent dye that accumulates in lamellar bodies. (H) Phase-contrast image of same field as in G. (I) G and H merged. (J) Green immunostaining shows proSP-C. (K) Red shows actin stained with phalloidin. (L) J and K merged. (M and N) Cryosection of human adult lung tissue was used as positive control for proSP-C (M) and phalloidin (N) staining. (O) M and N merged. Bars, 50 μm (A–E, G–I, and M–O) and 10 μm (J–L).
Figure 2.
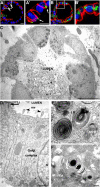
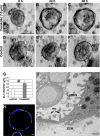
ALCs are well polarized. (A) Confocal micrographs of 4-d ALCs showed the basolateral marker β-catenin stained red, and the tight junction marker ZO-1 stained green. Note that ZO-1 staining was observed at cell–cell contacts facing the lumen (white arrow). (B) ALCs stained green for the Golgi marker GM130 and red for actin. GM130 was located laterally, whereas actin was localized at all plasma membranes, yet enriched at the luminal surface. A′ and B′ represent high magnification of regions indicated in A and B, respectively. (C) Electron micrographs showed cuboidal cells containing lamellar bodies; the lumen was filled with secreted lamellar bodies. (D–F) Shown is evidence of epithelial polarity and typical AT II characteristics, including numerous microvilli (arrowheads) facing the luminal surface and a tight junction structure (arrow) that was evident at cell–cell contacts near the lumen (D). The Golgi complex was between the nucleus (N) and a lateral surface. (E) Membrane-bounded LB and MVB were evident. Lamellar body contents that have been secreted from AT II cells were seen rearranged into a tubular myelin (TM) structure, which is a unique, secreted form a of surfactant protein having a lattice-like structure (F). Bars, 10 μm (A–C), 1 μm (D), and 200 nm (E and F).
Figure 3.
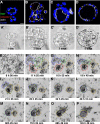
Confocal images show cell aggregation contributes to ALC formation. AT II cells cultured for 1, 2, 3, or 5 d (A–D, respectively) in Matrigel were stained with Ki67 (green), cleaved caspase 3 (red), actin (white), and nuclei (blue). (A′–D′) Phase-contrast images corresponding to A–D. (E–P) Shown are selected phase-contrast, time-lapse images of live cells taken 8–137 h (times indicated in each frame) after plating in Matrigel culture. (F–I) By 24 h, cells had aggregated into small clusters. (I–P) The multicellular structures in F–I aggregated further and finally became a structure having a single lumen. See Supplemental Material, Video 2, right panel. Bars, 10 μm (A–D′) and 50 μm (E–P).
Figure 4.
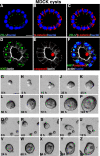
MDCK cells form cysts in Matrigel. (A–C) Confocal images of 5-d-old MDCK cysts grown in 2% Matrigel showed the basolateral marker β-catenin stained red, and the tight junction marker ZO-1 stained green. Note that ZO-1 staining was observed at cell–cell contacts facing the lumen. (D–F) Immunostaining with Ki67 (green in D and cyan in F when merged with nuclei in blue) and cleaved caspase 3 (red in E and F) in MDCK cysts. (G–P) Representative live images of a single MDCK cell, cultured in Matrigel for 5 d, generating a clonal cyst. (Q–Z) show still frames of live images of multiple individual MDCK cells aggregating and proliferating to form one cyst. Also see Supplemental Material, Video 3. Bars, 10 μm (A–F) and 20 μm (G–Z).
Figure 5.
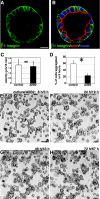
β1-Integrin is required for cell aggregation. (A) Green immunofluorescent staining of β1-integrin revealed that it was localized at the ALC basolateral surface. (B) The green staining in A is shown merged with red actin staining and blue nuclear staining. (C) Individual AT II cells in Matrigel in the presence of AIIB2 moved at an average speed of 1.7 μm/15 min. Control cells moved at 1.8 μm/15 min. ns, no significant difference between the two means. (D) Sixty one percent of individual cells adhered to other cells during the 6-h interval starting from 8 h after plating. In the presence of AIIB2, only 25% of cells adhered; the majority remained as individual cells; *, p < 0.01 (Student's t test). (E–H) Representative time-lapse images of AIIB2-treated cells. Note that even after 3-d culture, cells only formed small clusters: many remained single. Bars, 10 μm (A and B) and 50 μm (E–H).
Figure 6.
Forskolin treatment expands ALCs. Addition of 10 μM forskolin to ALCs after 3 d in culture increased ALC size within 24 h: the central lumen expanded and became more evident. (A–C) Phase-contrast images of cysts 0, 24, and 48 h after forskolin addition. (D–F) Over the same time interval, control ALCs remained the same diameter. (G) Forty-eight hours after forskolin treatment average ALC diameter increased from 60 μm (control) to 95 μm. *, p < 0.01. (H) This confocal image shows forskolin-treated ALCs stained green for Ki67, red for activated caspase 3, white for actin, and blue for nuclei; no significant Ki67- or caspase 3-positive cells were seen, whereas cells became thinner. (I) Transmission electron micrograph showing a portion of a cell within an ALC that had been exposed to forskolin for 24 h. The cell still contained LB. Microvilli (mv) were observed at the luminal surface, and some parts of the cell were very thin: <1 μm distance from apical to basolateral surface. Bars, 50 μm (A–F), 10 μm (H), and 1 μm (I).
Figure 7.
Schematic illustrates events during ALC formation and the blocking effect of β1-integrin. AT II cells in Matrigel begin aggregating 0.5 d after plating: cells formed small clusters by day 1. Further aggregation followed, and by 3–4 d polarized ALCs had formed. Blocking β1-integrin inhibited cell aggregation. Cells frequently collided with neighboring cells but largely failed to adhere.
Similar articles
- The microenvironmental determinants for kidney epithelial cyst morphogenesis.
Guo Q, Xia B, Moshiach S, Xu C, Jiang Y, Chen Y, Sun Y, Lahti JM, Zhang XA. Guo Q, et al. Eur J Cell Biol. 2008 Apr;87(4):251-66. doi: 10.1016/j.ejcb.2007.11.004. Epub 2008 Jan 8. Eur J Cell Biol. 2008. PMID: 18191498 Free PMC article. - Epithelial-mesenchymal co-culture model for studying alveolar morphogenesis.
Greer RM, Miller JD, Okoh VO, Halloran BA, Prince LS. Greer RM, et al. Organogenesis. 2014;10(4):340-9. doi: 10.4161/org.29198. Epub 2014 Oct 31. Organogenesis. 2014. PMID: 25482312 Free PMC article. - Developing fetal alveolar epithelial cells secrete fluid in primary culture.
McCray PB Jr, Welsh MJ. McCray PB Jr, et al. Am J Physiol. 1991 Jun;260(6 Pt 1):L494-500. doi: 10.1152/ajplung.1991.260.6.L494. Am J Physiol. 1991. PMID: 1711786 - Regulation of intracellular pH in alveolar epithelial cells.
Lubman RL, Crandall ED. Lubman RL, et al. Am J Physiol. 1992 Jan;262(1 Pt 1):L1-14. doi: 10.1152/ajplung.1992.262.1.L1. Am J Physiol. 1992. PMID: 1310224 Review. - [Regulation of alveolar type II cell proliferation and surfactant gene expression].
Sugahara K. Sugahara K. Nihon Kyobu Shikkan Gakkai Zasshi. 1994 Dec;32 Suppl:73-8. Nihon Kyobu Shikkan Gakkai Zasshi. 1994. PMID: 7602847 Review. Japanese.
Cited by
- Multipotent capacity of immortalized human bronchial epithelial cells.
Delgado O, Kaisani AA, Spinola M, Xie XJ, Batten KG, Minna JD, Wright WE, Shay JW. Delgado O, et al. PLoS One. 2011;6(7):e22023. doi: 10.1371/journal.pone.0022023. Epub 2011 Jul 7. PLoS One. 2011. PMID: 21760947 Free PMC article. - The proto-oncoprotein SYT (SS18) controls ATP release and regulates cyst formation by polarized MDCK cells.
Chittezhath M, Frump AL, Jourquin J, Lobdell N, Eid JE. Chittezhath M, et al. Exp Cell Res. 2008 Nov 15;314(19):3551-62. doi: 10.1016/j.yexcr.2008.09.006. Epub 2008 Sep 20. Exp Cell Res. 2008. PMID: 18835266 Free PMC article. - Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake.
Salvi AM, Bays JL, Mackin SR, Mege RM, DeMali KA. Salvi AM, et al. Nat Cell Biol. 2021 May;23(5):457-466. doi: 10.1038/s41556-021-00677-y. Epub 2021 May 10. Nat Cell Biol. 2021. PMID: 33972734 Free PMC article. - Rho activation drives luminal collapse and eversion in epithelial acini.
Narayanan V, Purkayastha P, Yu B, Pendyala K, Chukkapalli S, Cabe JI, Dickinson RB, Conway DE, Lele TP. Narayanan V, et al. Biophys J. 2023 Sep 19;122(18):3630-3645. doi: 10.1016/j.bpj.2023.01.005. Epub 2023 Jan 7. Biophys J. 2023. PMID: 36617192 Free PMC article. - Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration.
Andrew DJ, Ewald AJ. Andrew DJ, et al. Dev Biol. 2010 May 1;341(1):34-55. doi: 10.1016/j.ydbio.2009.09.024. Epub 2009 Sep 22. Dev Biol. 2010. PMID: 19778532 Free PMC article. Review.
References
- Debnath J., Brugge J. S. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer. 2005;5:675–688. - PubMed
- Debnath J., Mills K. R., Collins N. L., Reginato M. J., Muthuswamy S. K., Brugge J. S. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. - PubMed
- Fang X., Song Y., Hirsch J., Galietta J., Pedemonte N., Zemans R., Dolganov G., Verkman A., Matthay M. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am. J. Physiol. 2006;290:L242–L249. - PubMed
- Giancotti F. G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-51856/HL/NHLBI NIH HHS/United States
- P01 AI053194/AI/NIAID NIH HHS/United States
- HL-51854/HL/NHLBI NIH HHS/United States
- R01 HL051854/HL/NHLBI NIH HHS/United States
- AI-053194/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources