Transcription termination within the iron transport-biosynthesis operon of Vibrio anguillarum requires an antisense RNA - PubMed (original) (raw)
Transcription termination within the iron transport-biosynthesis operon of Vibrio anguillarum requires an antisense RNA
Michiel Stork et al. J Bacteriol. 2007 May.
Abstract
The iron transport-biosynthesis (ITB) operon in Vibrio anguillarum includes four genes for ferric siderophore transport, fatD, -C, -B, and -A, and two genes for siderophore biosynthesis, angR and angT. This cluster plays an important role in the virulence mechanisms of this bacterium. Despite being part of the same polycistronic mRNA, the relative levels of transcription for the fat portion and for the whole ITB message differ profoundly, the levels of the fat transcript being about 17-fold higher. Using S1 nuclease mapping, lacZ transcriptional fusions, and in vitro studies, we were able to show that the differential gene expression within the ITB operon is due to termination of transcription between the fatA and angR genes, although a few transcripts proceeded beyond the termination site to the end of this operon. This termination process requires a 427-nucleotide antisense RNA that spans the intergenic region and acts as a novel transcriptional terminator.
Figures
FIG. 1.
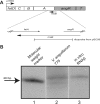
Genetic organization of the iron transport and biosynthesis operon on the pJM1 plasmid and Northern blot analysis of RNAβ. Panel A shows the operon organization of the ITB operon. The location of the probe derived from pSC59 is also shown. (B) Northern blot analysis. Lane 1, molecular weight marker; lane 2, RNA from V. anguillarum 775 grown in CM9 minimal medium; lane 3, in vitro-synthesized RNAβ. The lanes are from the same gel, but shown are different exposure times for lanes 1 and 3, since at the exposure time of lane 2, these were saturating the film.
FIG. 2.
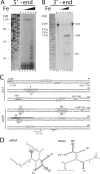
(A and B) Mapping of the 5′ end and 3′ end of RNAβ. (A) Primer extension analyses was carried out to determine the 5′ end of RNAβ. The products that extended from the 32P-labeled primer (highlighted in gray in panel C) were analyzed in parallel with a known sequence marker (lanes GACT) by urea-PAGE (6%). Panel B shows the results of S1 mapping analysis, which was performed with a 245-nt 32P-labeled riboprobe containing the 171-nt AflIII-NheI DNA fragment from the _fatA_-angR intergenic region (see panel C). The riboprobe without S1 nuclease treatment is shown in lanes U, alongside a known sequence marker (lanes TCAG). In both panels, the supplements to the medium were as follows: lane 1, 3 μM EDDA; lane 2, none; and lanes 3 to 6, ferric ammonium citrate at concentrations of 2 μg/ml (lane 3), 4 μg/ml (lane 4), 8 μg/ml (lane 5), and 16 μg/ml (lane 6). Lanes marked with E are empty. (C) The nucleotide sequence of the region encompassing the fatA 3′ end and angR 5′ end is shown. The 5′ end (+1) and both 3′ ends of RNAβ and the putative −10 promoter sequence for RNAβ are indicated. Boxes contain the nucleotides of the loops of the stem-loop structures shown in panel D. (D) The putative stem-loop structures were generated using the computer program FoldRNA, GCG (University of Wisconsin). The complementary stem-loop structures (I-I*, II-II*, III-III*, and IV-IV*) are darkly shaded. Position 1 in the sense RNA structure corresponds to the first nucleotide in the AflIII site shown in the sequence in panel C.
FIG. 3.
Analysis of the expression levels of the _fatA_-angR intergenic region mRNA. Panel A shows the RNase protection experiment carried out with the total RNA preparations isolated from strain 775 grown under iron-rich (lane 1 [2 μg/ml ferric ammonium citrate]) and low-iron (lane 2 [2 μM EDDA]) conditions. Panel B shows the real-time quantitative PCR results in change (fold) in expression.
FIG. 4.
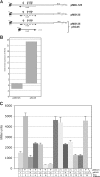
β-Galactosidase assay of transcriptional fusions of the intergenic region. (A) Schematic representation of the lacZ transcriptional fusions used in this study with the position of the sense mRNA stem-loops and the location of the rnaB gene. (B) Expression levels of RNAβ in pMS125 and pSC45 compared to pMDL125 as measured by real-time quantitative PCR. (C) Measurements of transcription termination using lacZ fusions. Shown are the average β-galactosidase activities (including the error) in Miller units of five independent experiments performed in triplicate for each strain. Under each bar is indicated what plasmids are present in V. anguillarum. L1C indicates that the bases in loop I have been changed to their complementary base, L2C indicates the complementary bases in loop II, and so on. + indicates that the plasmid is present as listed on the side, and − indicates that this particular plasmid is not present.
FIG. 5.
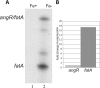
3′-end and 5′-end S1 mapping of the fatA-angR intergenic region. (A) Scheme of the expected banding patterns in the S1-treated samples if the difference in expression is the result of processing of a large mRNA including fatA-angR or transcription termination at a site between fatA and angR (see text for details). Asterisks indicate the position of the radioactive label. Please note that the order of the genes is depicted opposite from those for the other figures in this study. Panel B shows the actual results of the 5′- and 3′-end S1 mapping experiments using a 32P-labeled DNA probe and total RNA obtained from V. anguillarum 775 grown under iron-rich (lanes 1 and 3 [2 μg/ml ferric ammonium citrate]) and low-iron (lanes 2 and 4 [2 μM EDDA]) conditions.
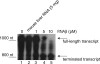
FIG. 6.
In vitro termination assay for RNAβ. We used E. coli RNA polymerase saturated with σ70, [α-32P]ATP and no addition (lane 1), 5 μg (20 pM) mouse liver RNA (lane 2), 1 pM RNAβ (lane 3), 5 pM RNAβ (lane 4), and 10 pM RNAβ (lane 5).
Similar articles
- Secondary structure of antisense RNAβ, an internal transcriptional terminator of the plasmid-encoded iron transport-biosynthesis operon of Vibrio anguillarum.
McIntosh-Tolle D, Stork M, Alice A, Crosa JH. McIntosh-Tolle D, et al. Biometals. 2012 Jun;25(3):577-86. doi: 10.1007/s10534-012-9542-x. Epub 2012 Apr 11. Biometals. 2012. PMID: 22491898 - Plasmid-mediated iron uptake and virulence in Vibrio anguillarum.
Stork M, Di Lorenzo M, Welch TJ, Crosa LM, Crosa JH. Stork M, et al. Plasmid. 2002 Nov;48(3):222-8. doi: 10.1016/s0147-619x(02)00111-7. Plasmid. 2002. PMID: 12460538 - Characterization of the angR gene of Vibrio anguillarum: essential role in virulence.
Wertheimer AM, Verweij W, Chen Q, Crosa LM, Nagasawa M, Tolmasky ME, Actis LA, Crosa JH. Wertheimer AM, et al. Infect Immun. 1999 Dec;67(12):6496-509. doi: 10.1128/IAI.67.12.6496-6509.1999. Infect Immun. 1999. PMID: 10569768 Free PMC article. - Biosynthetic and regulatory elements involved in the production of the siderophore vanchrobactin in Vibrio anguillarum.
Balado M, Osorio CR, Lemos ML. Balado M, et al. Microbiology (Reading). 2008 May;154(Pt 5):1400-1413. doi: 10.1099/mic.0.2008/016618-0. Microbiology (Reading). 2008. PMID: 18451049 - Characterization of ferric-anguibactin transport in Vibrio anguillarum.
López CS, Crosa JH. López CS, et al. Biometals. 2007 Jun;20(3-4):393-403. doi: 10.1007/s10534-007-9084-9. Epub 2007 Feb 8. Biometals. 2007. PMID: 17287889 Review.
Cited by
- Regulatory Interplay between RNase III and Antisense RNAs in E. coli: the Case of AsflhD and FlhD, Component of the Master Regulator of Motility.
Lejars M, Caillet J, Solchaga-Flores E, Guillier M, Plumbridge J, Hajnsdorf E. Lejars M, et al. mBio. 2022 Oct 26;13(5):e0098122. doi: 10.1128/mbio.00981-22. Epub 2022 Aug 24. mBio. 2022. PMID: 36000733 Free PMC article. - Integrated lncRNA function upon genomic and epigenomic regulation.
Herman AB, Tsitsipatis D, Gorospe M. Herman AB, et al. Mol Cell. 2022 Jun 16;82(12):2252-2266. doi: 10.1016/j.molcel.2022.05.027. Mol Cell. 2022. PMID: 35714586 Free PMC article. Review. - Diversity and Versatility in Small RNA-Mediated Regulation in Bacterial Pathogens.
Felden B, Augagneur Y. Felden B, et al. Front Microbiol. 2021 Aug 10;12:719977. doi: 10.3389/fmicb.2021.719977. eCollection 2021. Front Microbiol. 2021. PMID: 34447363 Free PMC article. Review. - Epigenetic Regulation of Alternative Splicing: How LncRNAs Tailor the Message.
Pisignano G, Ladomery M. Pisignano G, et al. Noncoding RNA. 2021 Mar 11;7(1):21. doi: 10.3390/ncrna7010021. Noncoding RNA. 2021. PMID: 33799493 Free PMC article. Review. - RNA-Dependent Regulation of Virulence in Pathogenic Bacteria.
Chakravarty S, Massé E. Chakravarty S, et al. Front Cell Infect Microbiol. 2019 Oct 9;9:337. doi: 10.3389/fcimb.2019.00337. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31649894 Free PMC article. Review.
References
- Actis, L. A., M. E. Tolmasky, D. H. Farrell, and J. H. Crosa. 1988. Genetic and molecular characterization of essential components of the Vibrio anguillarum plasmid-mediated iron-transport system. J. Biol. Chem. 263:2853-2860. - PubMed
- Argaman, L., R. Hershberg, J. Vogel, G. Bejerano, E. G. Wagner, H. Margalit, and S. Altuvia. 2001. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr. Biol. 11:941-950. - PubMed
- Bagdasarian, M., R. Lurz, B. Ruckert, F. C. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237-247. - PubMed
- Balaban, N., and R. P. Novick. 1995. Translation of RNAIII, the Staphylococcus aureus agr regulatory RNA molecule, can be activated by a 3′-end deletion. FEMS Microbiol. Lett. 133:155-161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources