Mitochondrial-targeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death - PubMed (original) (raw)
Mitochondrial-targeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death
Paramita Mookherjee et al. J Cell Biochem. 2007.
Abstract
Akt is a serine/threonine protein kinase that plays a vital role in promoting cellular survival. Predominantly cytosolic, upon stimulation with growth-factors or stress, active Akt translocates into mitochondria, but the functions of Akt in mitochondria are not yet fully understood. Mitochondria play a central role in apoptotic pathways and given Akt's functions in the cytoplasm, Akt in mitochondria may help preserve mitochondrial integrity during cellular stress. To test if the translocation of Akt into mitochondria is neuroprotective, adenoviral vectors expressing a constitutively active Akt, Ad-HA-Akt (DD), and a constitutively active Akt with a mitochondrial targeting signal, Ad-Mito-HA-Akt (DD), were generated. Human SH-SY5Y neuroblastoma cells expressing the adenoviral constructs were treated with staurosporine to initiate intrinsic apoptotic cell death and several aspects of the mitochondrial apoptotic pathway were evaluated. Expression of active Akt targeted to mitochondria was found to be sufficient to significantly reduce staurosporine-induced activation of caspase-3 and caspase-9, the release of cytochrome c from mitochondria, and Bax oligomerization at mitochondria. These findings demonstrate that intramitochondrial active Akt results in efficient protection against apoptotic signaling.
Figures
Fig. 1
Structure of HA-Akt constructs. The constructs used have an N-terminal HA-tag (diagonal lines) and the mitochondrial-targeted constructs have an N-terminal mitochondrial targeting signal (black). Using site-directed mutagenesis, the phosphorylation sites (T308 and S473) of HA-tagged Akt were mutated to aspartic acid residues (D308 and D473) to generate the constitutively active constructs.
Fig. 2
Expression and localization of HA-Akt constructs. A: Cell lysates of CHO cells transiently transfected with empty vector (pcDNA3.1; Ctl) or the HA-Akt constructs (wildtype,wt; constitutively active,DD) were collected and subjected to SDS–PAGE, followed by Western blot analysis with anti-HA antibody to determine expression level of the constructs. B: CHO cells transiently transfected with the HA-Akt constructs were separated into cytosolic (C) and mitochondrial (M) fractions and immunoblotted with the following antibodies: α-tubulin (cytosolic protein), ATP synthase β-subunit (mitochondrial protein), and anti-HA.
Fig. 3
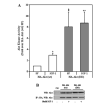
Akt kinase activity of HA-Akt (wt) and HA-Akt (DD) constructs. A: CHO cells transiently transfected with HA-Akt constructs were serum starved overnight and incubated in the absence (serum free [_SF_]) or presence (IGF-1) of 10 nM IGF-1 for 15 min. Cell lysates were collected, immunoprecipitated with an antibody to HA and Akt kinase activity was determined by in vitro kinase assay. The data are presented as mean ± SEM, n = 3 independent experiments; *, P < 0.05 versus HA-Akt (wt) SF; ‡, P < 0.001 versus HA-Akt (wt) SF; **, P < 0.005 versus HA-Akt (wt) SF (two-tailed, paired Student’s _t_-test). B: Representative immunoblot of cells transiently transfected with empty vector (pcDNA3.1; Ctl) or the HA-Akt constructs (upper panel). Representative immunoblot of HA immunoprecipitates probed with a polyclonal Akt antibody (lower panel).
Fig. 4
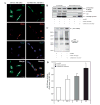
Ad-Mito-HA-Akt (DD) colocalizes with mitochondria and exhibits constitutive activation. A: SH-SY5Y cells seeded on chamber sides were infected with Ad-HA-Akt (DD) or Ad-Mito-HA-Akt (DD) and serum starved overnight. Cells were first loaded with Mitotracker Red CM-H2TMRos (Mito; red) to label mitochondria then fixed and labeled with the monoclonal anti-HA antibody (HA; green) to visualize the Akt adenoviral constructs and Dapi (Dapi; blue) to counterstain the nuclei. B: Homogenates and mitochondrial fractions from SH-SY5Y cells infected overnight with the indicated adenoviral constructs were immunoblotted for ATP synthase β-subunit (mitochondrial protein), α-tubulin (cytosolic protein), and Akt. C: SH-SY5Y cells infected with the designated adenoviral constructs were serum starved overnight, mitochondrial fractions prepared and probed for GSK3β phospho-Ser9 (pSer9-GSK3β) and phospho-Akt Substrate (PAS). D: SH-SY5Y cells infected with designated adenoviral constructs were serum starved overnight prior to incubation in the absence (serum free [_SF_]) or presence (IGF-1) of 10 nM IGF-1 for 15 min followed by preparation of mitochondria. Akt was immunoprecipitated from the mitochondrial fractions, and Akt kinase activity was determined by an in vitro kinase assay. The data are presented as mean ± SEM, n = 5 independent experiments; *, P < 0.05 versus Ad-GFP SF; **, P < 0.005 versus Ad-GFP SF (two-tailed, paired Student’s _t_-test).
Fig. 5
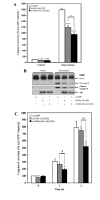
Mitochondrial-targeted active Akt protects SH-SY5Y cells from staurosporine-induced caspase activation. SH-SY5Y cells infected with the designated adenoviral constructs were serum starved overnight and incubated in the absence (control) or presence (staurosporine) of 0.5 μM staurosporine for 2 h (caspase-3 assay and PARP) or 0–2 h (caspase-9 activity). A: Caspase-3 activity. The presence of Ad-HA-Akt (DD) attenuated caspase-3 activation in response to staurosporine, with a significantly greater decrease being observed with Ad-Mito-HA-Akt (DD). The data are presented as mean ± SEM, n = 7 independent experiments; *, P < 0.05; ***, P < 0.0005 (one-way ANOVA). B: Representative blots showing the presence of cleaved PARP, cleaved caspase-3 and Akt. C: Caspase-9 activity. The presence of Ad-HA-Akt (DD) attenuated caspase-9 activation in response to staurosporine, with a significantly greater decrease being observed with Ad-Mito-HA-Akt (DD). The data are presented as mean ± SEM, n = 10 independent experiments; *, P < 0.05; †, P < 0.001; **, P <0.005 (two-tailed, paired Student’s _t_-test).
Fig. 6
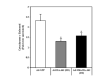
Active Akt reduces cytochrome c released from mitochondria of SH-SY5Y cells treated with staurosporine. SH-SY5Y cells infected with the designated adenoviral constructs were serum starved overnight and treated ± 0.5 μM staurosporine for 2 h. The amount of cytochrome c release from mitochondria was determined by ELISA. The data are presented as mean ± SEM, n = 6 independent experiments; *, P < 0.05 versus staurosporine-treated Ad-GFP (one-way ANOVA).
Fig. 7
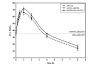
Mitochondrial membrane potential is not altered by the presence of active Akt in SH-SY5Y cells treated with staurosporine. SH-SY5Y cells infected with the designated adenoviral constructs were serum starved overnight and incubated with 0.5 μM staurosporine for the times indicated. The ratio was obtained by dividing JC-1 aggregate fluorescence by monomer fluorescence. The data are presented as mean ± SEM, n = 8 independent experiments.
Fig. 8
Mitochondrial-targeted active Akt reduces Bax oligomerization in response to staurosporine treatment. SH-SY5Y cells infected with the designated adenoviral constructs were serum starved overnight and incubated in the absence (control) or presence (staurosporine) of 0.5 μM staurosporine for 2 h. The oligomerization of Bax was determined by crosslinking the mitochondrial followed by immunoblotting with a Bax antibody.
Similar articles
- GSK3 promotes arsenite-induced apoptosis via facilitation of mitochondria disruption.
Watcharasit P, Thiantanawat A, Satayavivad J. Watcharasit P, et al. J Appl Toxicol. 2008 May;28(4):466-74. doi: 10.1002/jat.1296. J Appl Toxicol. 2008. PMID: 17849503 - Cx43 Mediates Resistance against MPP⁺-Induced Apoptosis in SH-SY5Y Neuroblastoma Cells via Modulating the Mitochondrial Apoptosis Pathway.
Kim IS, Ganesan P, Choi DK. Kim IS, et al. Int J Mol Sci. 2016 Nov 1;17(11):1819. doi: 10.3390/ijms17111819. Int J Mol Sci. 2016. PMID: 27809287 Free PMC article. - Pharmacological inhibition of GSK3 attenuates DNA damage-induced apoptosis via reduction of p53 mitochondrial translocation and Bax oligomerization in neuroblastoma SH-SY5Y cells.
Ngok-Ngam P, Watcharasit P, Thiantanawat A, Satayavivad J. Ngok-Ngam P, et al. Cell Mol Biol Lett. 2013 Mar;18(1):58-74. doi: 10.2478/s11658-012-0039-y. Epub 2012 Nov 16. Cell Mol Biol Lett. 2013. PMID: 23161404 Free PMC article. - Cytochrome c: the Achilles' heel in apoptosis.
Kulikov AV, Shilov ES, Mufazalov IA, Gogvadze V, Nedospasov SA, Zhivotovsky B. Kulikov AV, et al. Cell Mol Life Sci. 2012 Jun;69(11):1787-97. doi: 10.1007/s00018-011-0895-z. Epub 2011 Dec 17. Cell Mol Life Sci. 2012. PMID: 22179840 Free PMC article. Review.
Cited by
- Midazolam alleviates cellular senescence in SH-SY5Y neuronal cells in Alzheimer's disease.
Wang P, Wang P, Luan H, Wu Y, Chen Y. Wang P, et al. Brain Behav. 2023 Jan;13(1):e2822. doi: 10.1002/brb3.2822. Epub 2022 Nov 28. Brain Behav. 2023. PMID: 36444490 Free PMC article. - Selective modulation of subtype III IP₃R by Akt regulates ER Ca²⁺ release and apoptosis.
Marchi S, Marinello M, Bononi A, Bonora M, Giorgi C, Rimessi A, Pinton P. Marchi S, et al. Cell Death Dis. 2012 May 3;3(5):e304. doi: 10.1038/cddis.2012.45. Cell Death Dis. 2012. PMID: 22552281 Free PMC article. - Mitochondrial Ca(2+) and apoptosis.
Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P. Giorgi C, et al. Cell Calcium. 2012 Jul;52(1):36-43. doi: 10.1016/j.ceca.2012.02.008. Epub 2012 Apr 3. Cell Calcium. 2012. PMID: 22480931 Free PMC article. Review. - Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling.
Packer L, Cadenas E. Packer L, et al. J Clin Biochem Nutr. 2011 Jan;48(1):26-32. doi: 10.3164/jcbn.11-005FR. Epub 2010 Dec 29. J Clin Biochem Nutr. 2011. PMID: 21297908 Free PMC article. - PI3K/Akt pathway activation attenuates the cytotoxic effect of methyl jasmonate toward sarcoma cells.
Elia U, Flescher E. Elia U, et al. Neoplasia. 2008 Nov;10(11):1303-13. doi: 10.1593/neo.08636. Neoplasia. 2008. PMID: 18953440 Free PMC article.
References
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. - PubMed
- Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: The role of the PH domain. Oncogene. 1998;17:313–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS037768-04/NS/NINDS NIH HHS/United States
- NS037768/NS/NINDS NIH HHS/United States
- R01 NS037768-09/NS/NINDS NIH HHS/United States
- R01 NS051279/NS/NINDS NIH HHS/United States
- R01 NS037768/NS/NINDS NIH HHS/United States
- NS051279/NS/NINDS NIH HHS/United States
- R01 MH038752-23/MH/NIMH NIH HHS/United States
- R01 MH038752/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials