Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease - PubMed (original) (raw)
Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease
Jennifer L Orthmann-Murphy et al. Mol Cell Neurosci. 2007 Apr.
Abstract
Recessive mutations in GJA12/Cx47, the gene encoding the gap junction protein connexin47 (Cx47), cause Pelizaeus-Merzbacher-like disease (PMLD), which is characterized by severe CNS dysmyelination. Three missense PMLD mutations, P87S, Y269D and M283T, were expressed in communication-incompetent HeLa cells, and in each case the mutant proteins appeared to at least partially accumulate in the ER. Cells expressing each mutant did not pass Lucifer Yellow or neurobiotin in scrape loading assays, in contrast to robust transfer in cells expressing wild type Cx47. Dual whole-cell patch clamping of transfected Neuro2A cells demonstrated that none of the mutants formed functional channels, in contrast to wild type Cx47. Immunostaining sections of primate brains demonstrated that oligodendrocytes express Cx47, which is primarily localized to their cell bodies. Thus, the Cx47 mutants associated with PMLD likely disrupt the gap junction coupling between astrocytes and oligodendrocytes.
Figures
Figure 1. Expression of GJA12/Cx47 missense mutations associated with PMLD
(A) This is a schematic drawing of human Cx47 that illustrates the position and nature of mutations associated with PMLD; note the four missense mutations P87S, G233S, Y269D, and M283T, as well as P128frameshift, R237stop, and L278frameshift, P327frameshift (Uhlenberg et al. 2004; Bugiani et al. 2006). The positions of the transmembrane domains are based on the work of Yeager and Nicholson (Yeager and Nicholson 1996). (B) Immunoblot analysis of Cx47. The immunoblot was hybridized with an affinity-purified rabbit antiserum against mouse Cx47. Untransfected HeLa parental cells and cells transfected with vector alone do not express Cx47; the specific band corresponding to 47 kDa was present in lysates collected from bulk-selected cells expressing WT Cx47 as well as the P87S, Y269D, and M283T mutants (arrowhead). The asterisk denotes a background band.
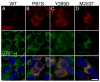
Figure 2. PMLD-associated Cx47 mutants are retained intracellularly and colocalized with an ER marker
These are deconvolved images of bulk-selected HeLa cells that express WT Cx47 or the indicated mutants, immunostained with a rabbit antiserum against mouse Cx47 (red) and a rat monoclonal against the ER chaperone GRP94 (green), and counterstained with DAPI (blue). Note that WT Cx47 forms gap junction plaques at cell borders, whereas Cx47 mutants are mainly intracellularly localized, except for M283T, which also forms puncta, mainly at cell borders (D). The intracellular Cx47-immunoreactivity of the mutants (B–D) colocalizes with GRP94. Scale bar: 10μm.
Figure 3. Disrupting the Golgi apparatus does not alter the localization of Cx47 mutants
These are confocal (untreated WT example only) or deconvolved images of bulk-selected HeLa cells that express WT Cx47 or the indicated mutants, immunostained with a rabbit antiserum against mouse Cx47 (red) and a mouse monoclonal antibody against a 58K Golgi protein (green). Incubation in brefeldin A (BFA) for 6 hours causes the redistribution of 58K from a polarized, perinuclear aggregation to a dispersed ER-like pattern of staining. BFA treatment does not affect the localization of P87S or Y269D, both of which are localized in the ER. BFA appears to reduce the number of gap junction plaques and increase the amount of intracellular Cx47-immunoreactivity in cells expressing WT Cx47, but has no apparent effect on cells expressing M283T. All images were acquired from the same experiment with the same exposure time for each channel (except for the untreated WT example). Scale bar: 10μm.
Figure 4. Intracellular Cx47 mutant protein is soluble in 1% Triton X-100
These are deconvolved images of bulk-selected HeLa cells that express WT Cx47 or the indicated mutants. The cells were incubated for 30 min in PBS containing 1% Triton X-100 (TX100), then immunostained with a rabbit antiserum against mouse Cx47 (red). The gap junction plaques formed by WT Cx47 and puncta of M283T are TX100-insoluble (A&D), whereas intracellular Cx47 of P87S (B), Y269D (C), and M283T (D) is TX100-soluble. All images were acquired from the same experiment with the same exposure time. Scale bar: 10μm.
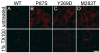
Figure 5. HeLa cells expressing Cx47 mutants are not dye-coupled
These are images of bulk-selected HeLa cells that express WT Cx47 or the indicated mutants, scrape loaded with Lucifer Yellow (LY, A-D) or neurobiotin (NB, E–H). Panels A–D show both epifluorescence and phase contrast images from live cells 5 min after scrape loading with LY. Panels E–H show cells that were scrape loaded in NB, then visualized with rhodamine-conjugated streptavidin (red) and counterstained with DAPI (blue). Only HeLa cells expressing WT Cx47 (A&E) show transfer of LY or NB. Scale bar: 20μm.
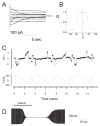
Figure 6. Functional properties of Cx47 in transiently transfected Neuro2A cells
(A) Representative current traces for homotypic human Cx47 channels. At rest, both cells were voltage clamped at 0 mV, then cell 2 was stepped in 20 mV increments from −100 to +100 mV, and junctional currents (Ij) were recorded from cell 1. As the absolute value of applied voltage increased, the rate of Ij decay increased, especially at 60, 80, and 100 mV. (B) The Gj-Vj relation for homotypic Cx47 channels. The average normalized steady state junctional conductance (Gj) in relation to the junctional voltage (Vj) was calculated from the current trace at each voltage. The solid line represents the fit of the data to Boltzmann distributions. Parameters (+Vj, −Vj); Gmin: 0.13, 0.14; Gmax: 0.98, 0.99; K: 5.8, 5.0; V0: 46.7, −44.5. Note that Gj decreases symmetrically as the absolute value of Vj increases. (C) The single channel conductance (non-normalized, gj) of Cx47. gj was determined during recovery from octanol treatment by repeatedly applying voltage ramps from +100 to −100 mV to one cell of a pair (bottom record), and measuring the transjunctional current of single channels in cell 2 (upper record). The gj of the fully open channel is ~53 pS, with a predominant residual conductance of ~8 pS. (D) Chemical gating of Cx47 channels. Ij was measured during application of repeated voltage ramps of ± 30 mV, before, during, and after bath application of octanol (2 mM). Note that the reduction of Ij by octanol was more rapid than the recovery.
Figure 7. Primate oligodendrocytes express Cx47
These are images of sections of rhesus monkey optic nerve, immunostained with mouse monoclonal antibodies against Cx47 (A–D) or Cx43 (E–F), a rabbit antisera against aspartoacylase (ASPA; A) or Cx43 (B), visualized with FITC- or TRITC- (A&B) or peroxidase-conjugated secondary antiserum (C–F), and counterstained with DAPI (A&B, merged panels). Cx47-immunoreactivity is diffusely dispersed within small cell bodies (A–B, green; C–D), with occasional gap junction plaques (arrowhead; D), and colocalizes with ASPA (A), a marker of oligodendrocytes. Cx43-immunoreactivity is found on astrocyte cell bodies and their proximal processes (E) and in gap junction plaques that are distributed throughout the optic nerve, including on small cell bodies that are likely to be oligodendrocytes (arrowheads, B&F). Scale bars: 10μm.
References
- Bergoffen J, Scherer SS, Wang S, Oronzi-Scott M, Bone L, Paul DL, Chen K, Lensch MW, Chance P, Fischbeck K. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993;262:2039–2042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS055284/NS/NINDS NIH HHS/United States
- T32 AG000255/AG/NIA NIH HHS/United States
- NS054363/NS/NINDS NIH HHS/United States
- NS42878/NS/NINDS NIH HHS/United States
- NS050345/NS/NINDS NIH HHS/United States
- F30 NS054363/NS/NINDS NIH HHS/United States
- AG 00255/AG/NIA NIH HHS/United States
- R01 NS050705/NS/NINDS NIH HHS/United States
- K02 NS050345/NS/NINDS NIH HHS/United States
- R01 NS043560/NS/NINDS NIH HHS/United States
- NS050705/NS/NINDS NIH HHS/United States
- R01 NS042878/NS/NINDS NIH HHS/United States
- NS043560/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous