Severe acute respiratory syndrome coronavirus accessory protein 6 is a virion-associated protein and is released from 6 protein-expressing cells - PubMed (original) (raw)
Severe acute respiratory syndrome coronavirus accessory protein 6 is a virion-associated protein and is released from 6 protein-expressing cells
Cheng Huang et al. J Virol. 2007 May.
Abstract
Analysis of severe acute respiratory syndrome coronavirus (SCoV) by either sucrose gradient equilibrium centrifugation or a virus capture assay using an anti-SCoV S protein antibody demonstrated that the SCoV 6 protein, which is one of the accessory proteins of SCoV, was incorporated into virus particles. Coexpression of the SCoV S, M, E, and 6 proteins was sufficient for incorporation of the 6 protein into virus-like particles. Cells transfected with plasmid expressing the 6 protein released SCoV 6 protein; however, infected cells released SCoV 6 protein only in association with SCoV particles.
Figures
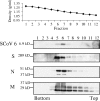
FIG. 1.
Association of the SCoV 6 protein with purified SCoV particles. Culture media from SCoV-infected Caco2 cells were inactivated by irradiation. Samples were clarified and partially purified by centrifugation through 20% sucrose cushions. Pellets were suspended in NTE buffer (100 mM NaCl, 10 mM Tris-HCl [pH 7.5], 1 mM EDTA) and purified by ultracentrifugation on a continuous 20 to 60% sucrose gradient by using a Beckman SW28 rotor. Twelve fractions were taken from the bottom. The purified viruses in each fraction were pelleted by centrifugation through a 20% sucrose cushion. Samples were dissolved in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 50 mM dithiothreitol) and separated on a 15% SDS-PAGE gel for detection of the SCoV 6 protein, on a 12% SDS-PAGE gel for the M and N proteins, or on an 8% SDS-PAGE gel for the S protein. Western blotting analysis using anti-SCoV 6 antibody (SCoV 6), anti-SCoV S protein antibody (S), anti-SCoV N antibody (N), and anti-SCoV M protein antibody (M) was performed to detect the SCoV 6, S, N, and M proteins, respectively.
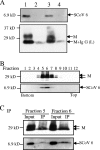
FIG. 2.
Results of the SCoV capture assay. (A) SCoV particles in clarified media from SCoV-infected Caco2 cells were pelleted down by centrifugation on a 20% sucrose cushion. The suspended pellets were immunoprecipitated by a nonspecific monoclonal mouse anti-H2K antibody (lane 2) or by a mouse monoclonal anti-SCoV S antibody (lane 3) to capture virions. Samples were examined with rabbit anti-SCoV 6 protein antibody (top panel) and anti-SCoV M antibody (bottom panel) by Western blot analysis. Lanes 1 and 4 represent a SCoV sample partially purified by a 20% sucrose cushion and supernatant from the SCoV samples after purification with anti-SCoV S protein antibody, respectively. A minor signal detected in lanes 2 and 4 represents the immunoglobulin G light chain, which comigrated with a fast-migrating major M protein signal in lane 3. (B) SCoV samples were purified by ultracentrifugation on a 20 to 60% sucrose gradient. Twelve fractions were collected from bottom to top and subjected to Western blot analysis to detect the SCoV M and 6 proteins. (C) Fractions 5 and 6 from panel B, containing the purified SCoV particles, were further subjected to the virus capture assay to detect virion-associated SCoV M protein and 6 protein. Input, pelleted samples from fractions 5 and 6; IP, sample obtained after immunoprecipitation.
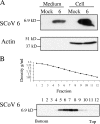
FIG. 3.
Release of the SCoV 6 protein from cells expressing the 6 protein. (A) 293T cells were transfected with empty-vector pCAGGS (mock) or pCAGGS-6 (6). At 48 h posttransfection, supernatants from transfected cells were clarified, applied onto a 20% sucrose cushion, and centrifuged at 26,000 rpm for 3 h at 4°C. The pellets were suspended in 1× SDS-PAGE loading buffer (Medium). Cell lysates were prepared with 1× SDS-PAGE loading buffer (Cell). Samples were separated on a 15% SDS-PAGE gel and subjected to Western blot analysis with anti-SCoV 6 protein antibody (6). The membranes were reprobed with anti-actin antibody (Actin). (B) The supernatant of 293T cells expressing the 6 protein was collected 48 h after transfection and partially purified by centrifugation through a 20% sucrose cushion. The pellets were suspended in NTE buffer, loaded on a 20 to 60% continuous sucrose gradient, and centrifuged at 26,000 rpm for 18 h using a Beckman SW28 rotor. Twelve fractions were taken from the bottom, and the densities for each action were measured (upper panel). Each fraction was then diluted at least threefold with NTE buffer and was then centrifuged through a 20% sucrose cushion. The recovered pellets were suspended in 1× SDS-PAGE loading buffer and subjected to Western blot analysis with anti-SCoV 6 protein antibody (lower panel).
FIG. 4.
Assembly of SCoV 6 protein into VLPs. A mixture of plasmids, containing 9 μg of pCAGGS-S, 6 μg of pCAGGS-M, 4 μg of pCAGGS-E, and 0.5 μg of pCAGGS-6, was transfected into 293T cells (lanes 2, 3, and 4). As a control, cells were transfected with 19.5 μg of pCAGGS (lane 1). At 48 h posttransfection, culture media were harvested, and the released VLPs were pelleted by centrifugation through a 20% sucrose cushion. SCoV VLPs were resuspended in NTE buffer containing 0.3% of bovine serum albumin and immunoprecipitated with nonspecific monoclonal mouse anti-H2K antibody (lane 3) or mouse anti-SCoV S protein monoclonal antibody (NR-617) (lane 4). The captured SCoV VLPs (lanes 3 and 4) and cell lysates (lanes 1 and 2) were analyzed using Western blot analysis with anti-SCoV 6 protein antibody (top panel) and anti-M protein antibody (bottom panel).
Similar articles
- Severe acute respiratory syndrome coronavirus 3a protein is released in membranous structures from 3a protein-expressing cells and infected cells.
Huang C, Narayanan K, Ito N, Peters CJ, Makino S. Huang C, et al. J Virol. 2006 Jan;80(1):210-7. doi: 10.1128/JVI.80.1.210-217.2006. J Virol. 2006. PMID: 16352545 Free PMC article. - Severe acute respiratory syndrome coronavirus 7a accessory protein is a viral structural protein.
Huang C, Ito N, Tseng CT, Makino S. Huang C, et al. J Virol. 2006 Aug;80(15):7287-94. doi: 10.1128/JVI.00414-06. J Virol. 2006. PMID: 16840309 Free PMC article. - Two palmitylated cysteine residues of the severe acute respiratory syndrome coronavirus spike (S) protein are critical for S incorporation into virus-like particles, but not for M-S co-localization.
Ujike M, Huang C, Shirato K, Matsuyama S, Makino S, Taguchi F. Ujike M, et al. J Gen Virol. 2012 Apr;93(Pt 4):823-828. doi: 10.1099/vir.0.038091-0. Epub 2012 Jan 13. J Gen Virol. 2012. PMID: 22238235 - Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-protein interactions.
Li W, Wong SK, Li F, Kuhn JH, Huang IC, Choe H, Farzan M. Li W, et al. J Virol. 2006 May;80(9):4211-9. doi: 10.1128/JVI.80.9.4211-4219.2006. J Virol. 2006. PMID: 16611880 Free PMC article. Review. No abstract available. - SARS coronavirus accessory proteins.
Narayanan K, Huang C, Makino S. Narayanan K, et al. Virus Res. 2008 Apr;133(1):113-21. doi: 10.1016/j.virusres.2007.10.009. Epub 2007 Nov 28. Virus Res. 2008. PMID: 18045721 Free PMC article. Review.
Cited by
- Systematizing the genomic order and relatedness in the open reading frames (ORFs) of the coronaviruses.
Barik S. Barik S. Infect Genet Evol. 2021 Aug;92:104858. doi: 10.1016/j.meegid.2021.104858. Epub 2021 Apr 18. Infect Genet Evol. 2021. PMID: 33848683 Free PMC article. - SARS coronavirus 8b reduces viral replication by down-regulating E via an ubiquitin-independent proteasome pathway.
Keng CT, Akerström S, Leung CS, Poon LL, Peiris JS, Mirazimi A, Tan YJ. Keng CT, et al. Microbes Infect. 2011 Feb;13(2):179-88. doi: 10.1016/j.micinf.2010.10.017. Epub 2010 Oct 28. Microbes Infect. 2011. PMID: 21035562 Free PMC article. - Mechanisms of severe acute respiratory syndrome pathogenesis and innate immunomodulation.
Frieman M, Baric R. Frieman M, et al. Microbiol Mol Biol Rev. 2008 Dec;72(4):672-85, Table of Contents. doi: 10.1128/MMBR.00015-08. Microbiol Mol Biol Rev. 2008. PMID: 19052324 Free PMC article. Review. - Identification of potential inhibitors of coronavirus hemagglutinin-esterase using molecular docking, molecular dynamics simulation and binding free energy calculation.
Patel CN, Kumar SP, Pandya HA, Rawal RM. Patel CN, et al. Mol Divers. 2021 Feb;25(1):421-433. doi: 10.1007/s11030-020-10135-w. Epub 2020 Sep 29. Mol Divers. 2021. PMID: 32996011 Free PMC article. - Nonproductive exposure of PBMCs to SARS-CoV-2 induces cell-intrinsic innate immune responses.
Kazmierski J, Friedmann K, Postmus D, Emanuel J, Fischer C, Jansen J, Richter A, Bosquillon de Jarcy L, Schüler C, Sohn M, Sauer S, Drosten C, Saliba AE, Sander LE, Müller MA, Niemeyer D, Goffinet C. Kazmierski J, et al. Mol Syst Biol. 2022 Aug;18(8):e10961. doi: 10.15252/msb.202210961. Mol Syst Biol. 2022. PMID: 35975552 Free PMC article.
References
- Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI29984/AI/NIAID NIH HHS/United States
- N01-AI25489/AI/NIAID NIH HHS/United States
- R21 AI029984/AI/NIAID NIH HHS/United States
- R01 AI029984/AI/NIAID NIH HHS/United States
- N01 AI025489/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources