Site-directed transposon integration in human cells - PubMed (original) (raw)
Site-directed transposon integration in human cells
Stephen R Yant et al. Nucleic Acids Res. 2007.
Abstract
The Sleeping Beauty (SB) transposon is a promising gene transfer vector that integrates nonspecifically into host cell genomes. Herein, we attempt to direct transposon integration into predetermined DNA sites by coupling a site-specific DNA-binding domain (DBD) to the SB transposase. We engineered fusion proteins comprised of a hyperactive SB transposase (HSB5) joined via a variable-length linker to either end of the polydactyl zinc-finger protein E2C, which binds a unique sequence on human chromosome 17. Although DBD linkage to the C-terminus of SB abolished activity in a human cell transposition assay, the N-terminal addition of the E2C or Gal4 DBD did not. Molecular analyses indicated that these DBD-SB fusion proteins retained DNA-binding specificity for their respective substrate molecules and were capable of mediating bona fide transposition reactions. We also characterized transposon integrations in the presence of the E2C-SB fusion protein to determine its potential to target predefined DNA sites. Our results indicate that fusion protein-mediated tethering can effectively redirect transposon insertion site selection in human cells, but suggest that stable docking of integration complexes may also partially interfere with the cut-and-paste mechanism. These findings illustrate the feasibility of directed transposon integration and highlight potential means for future development.
Figures
Figure 1.
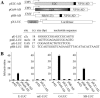
Fusion protein strategy to achieve site-directed transposon integration. (A) Reporter assay for monitoring DNA-binding specificity of candidate DNA-binding domains (DBD). Prospective DBDs were fused to the VP16 activation domain (AD) and expressed from the strong CMV promoter. These activator plasmids were co-transfected into HeLa cells together with a reporter plasmid containing a luciferase gene, a minimal promoter element, and five upstream binding sites for the DBDs (XXXXX) such that co-delivery of an appropriate activator and reporter plasmid results in activation of the downstream luciferase reporter gene. E2C, a synthetic polydactyl zinc-finger protein that recognizes the e2c target site; Gal4, the DNA-binding domain from the Gal4 protein that binds to upstream activator sequences (UAS); SB(N123), the SB transposase N-terminal 123 amino acid DNA-binding domain that binds to the SB transposon IDR (inner direct repeat). (B) DNA-binding specificity of independent protein domains within the context of human cells. Each graph displays luciferase activity relative to transfection with empty vector (pAD). Bars represent the average (mean ± st.dev.) obtained from three independent transfection experiments.
Figure 2.
Schematic overview of the fusion proteins described in this report. Each construct contains an enzymatic transposase domain fused to a site-specific DNA-binding domain protein. For the synthetic zinc-finger protein E2C, both possible configurations (N- and C-terminal fusions) were tested. HSB5, a hyperactive variant of the SB10 transposase; G59A, an alanine substitution mutation in the DNA-binding domain of SB that disrupts its ability to bind transposon DNA; E279A, an alanine substitution mutation in the catalytic core of SB that disrupts its excision and integration activity. Black boxes denote flexible inter-domain linkers of variable lengths (0–21 amino acids).
Figure 3.
Optimizing expression and activity of chimeric transposase proteins in human cells. (A) Upper panel, schematic overview of SB transposition assay. Lower panel, fusion protein activity in human cells. Fusion proteins containing variable numbers (n) of a flexible inter-domain (GGS)n peptide linker were tested for transpositional activity in HeLa cells. The (Gly–Gly–Ser)5 linker supporting the highest level of integration activity was designated L5 for the sake of simplicity. The average number of integration events obtained from three independent transfections is shown (mean ± st.dev.). (B) Western blot analysis of fusion protein expression. Transfected HeLa cells were harvested two days post-transfection, lysed and subjected to immunoblot analysis using a polyclonal antibody against the SB transposase. The right panel shows an attempt to normalize HSB5 and fusion protein expression in the cell by transfecting diminishing amounts of the HSB5 plasmid (1×, 0.1× or 0.05×) relative to fusion protein constructs. (C) Excision activity of chimeric transposases. HeLa cells were transfected with a neomycin-marked (neor) transposon plasmid together with plasmids encoding GFP, HSB5 transposase, or selected fusion proteins. Hirt DNA samples were prepared 2 days later and used as template in a series of nested PCR reactions. Transposon excision and subsequent DNA repair by the host enables the amplification of a diagnostic 253 bp PCR excision-and-repair product. (D) Sequence analysis of transposon excision and repair products generated from HSB5- and DBD-SB-mediated transposition. Target TA dinucleotides are capitalized. Number of events of each type are shown in parentheses.
Figure 4.
DNA-binding activity of the E2C-L5-SB fusion protein. (A) Analysis of E2C-L5-SB fusion protein DNA-binding activity by electro-mobility shift assay. N123-based peptides corresponding to truncated forms of E2C-L5-SB and E2C-L5-SB-G59A (control) fusion proteins were produced by in vitro transcription and translation, and then incubated with 32P-radiolabelled double-stranded DNA probes (IDR, e2c or mutant e2c). Protein–DNA complexes were formed in the presence and absence of excess unlabelled competitor DNA, resolved by electrophoresis through a gel, and visualized by autoradiography. The competitor in lane 8 is unlabelled IDR DNA. C1, fusion peptide–IDR complex; C2, fusion peptide–e2c complex; C3, fusion peptide/IDR/e2c trimeric complex. (B) Schematic of competition assay to monitor the DNA-binding activity of full-length hE2C-L5-SB and Gal4-L5-SB fusion proteins within human cells. HeLa cells were transfected with luciferase reporter plasmids together with limiting amounts of an activator plasmid encoding their respective trans-activator protein (hE2C-AD, Gal4-AD or SB(N123)-AD). Cells also received an excess of plasmids encoding various experimental and control proteins to test whether any could compete for protein binding at the target sites, thereby reducing the level of luciferase _trans_-activation in the cell. (C) Reporter assay. Each graph displays luciferase activation levels relative to transfection with a control vector (pc-GFP). Bars represent the average (mean ± st.dev.) obtained from three independent transfection experiments.
Figure 5.
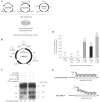
Site-directed transposition in human cells. (A) Schematic overview of plasmid-based assay for investigating site-directed transposition. Transposition was initiated by transfecting human HeLa cells with a plasmid encoding chimeric or unfused HSB5 transposase, together with a donor plasmid encoding a bleomycin-marked (zeor) transposon and a counter-selectable chloramphenicol-resistance (camr) gene. These plasmids were co-delivered with an ampicillin-resistant (ampr) target plasmid containing five tandem DBD recognition sequences and allowed to undergo transposition. Low-molecular weight plasmid DNA fractions were isolated 2 days later and transformed into DH10B E. coli. Replication of the R6K origin-containing donor plasmid is strictly dependent on expression of the pir1 gene product, which is absent in this bacterial strain. Ampr/zeor bacterial colonies were patched onto LB-camr plates to screen for inter-plasmid transposition events specific for the target plasmid (i.e. cams). Both pooled and clonal ampr/zeor/cams populations of bacteria were amplified, plasmid DNA isolated and the locations of transposon insertions relative to the target sites determined by restriction site analyses and DNA sequence analyses, respectively. (B) Target plasmid features. Positions of BglI and BglII restriction endonuclease recognition sites are shown, as are the sizes for each resulting DNA fragment. (C) Southern blot analysis of targeted integration. For each experimental condition, 500 ng of plasmid DNA isolated from pooled ampr/zeor/cams bacterial colonies (n = 43–51) was treated with BglI-BglII restriction enzymes. Samples were resolved on an agarose gel, transferred to nitrocellulose, hybridized to a 32P-radiolabelled probe corresponding to the left SB transposon inverted repeat, and resulting bands visualized upon autoradiography. In one instance, excess E2C DNA-binding domain was co-expressed with transposase protein to determine whether associated proteins could inhibit SB target site DNA capture. The lower band intensity under each experimental condition represents a qualitative assessment of the relative frequency of transposition of the 1.35 kb zeor-marked element into the 443 bp targeting window. (D) Targeted transposition frequencies. Recombinant target DNA molecules were isolated from individual ampr/zeor/cams colonies and sequenced using an internal transposon-specific primer. Bars denote the percentage of total integrations occurring within the 443-bp targeting window, whereas the numbers in parentheses denote the actual number of integrations analyzed in each group. (E) Flexing model for targeted transposition. Multiple changes in both DNA- and protein conformation are necessary to complete a fullcycle of transposition. A protein that remains too tightly bound to DNA, such as hE2C-L5-SB to the canonical e2c site, cannot efficiently catalyze these reactions. In the case of the mutant e2c site, however, only fingers 1 through 3 of hE2C-L5-SB retain the capacity for DNA-binding. This would essentially improve the flexibility of the transposase domain, which might enhance the acquisition and/or manipulation of neighboring target sites.
Similar articles
- Jumping Ahead with Sleeping Beauty: Mechanistic Insights into Cut-and-Paste Transposition.
Ochmann MT, Ivics Z. Ochmann MT, et al. Viruses. 2021 Jan 8;13(1):76. doi: 10.3390/v13010076. Viruses. 2021. PMID: 33429848 Free PMC article. Review. - Retargeting sleeping beauty transposon insertions by engineered zinc finger DNA-binding domains.
Voigt K, Gogol-Döring A, Miskey C, Chen W, Cathomen T, Izsvák Z, Ivics Z. Voigt K, et al. Mol Ther. 2012 Oct;20(10):1852-62. doi: 10.1038/mt.2012.126. Epub 2012 Jul 10. Mol Ther. 2012. PMID: 22776959 Free PMC article. - Targeted Sleeping Beauty transposition in human cells.
Ivics Z, Katzer A, Stüwe EE, Fiedler D, Knespel S, Izsvák Z. Ivics Z, et al. Mol Ther. 2007 Jun;15(6):1137-44. doi: 10.1038/sj.mt.6300169. Epub 2007 Apr 10. Mol Ther. 2007. PMID: 17426709 - Herpes simplex virus/Sleeping Beauty vector-based embryonic gene transfer using the HSB5 mutant: loss of apparent transposition hyperactivity in vivo.
de Silva S, Mastrangelo MA, Lotta LT Jr, Burris CA, Izsvák Z, Ivics Z, Bowers WJ. de Silva S, et al. Hum Gene Ther. 2010 Nov;21(11):1603-13. doi: 10.1089/hum.2010.062. Epub 2010 Oct 22. Hum Gene Ther. 2010. PMID: 20507234 Free PMC article. - Sleeping Beauty Transposition.
Ivics Z, Izsvák Z. Ivics Z, et al. Microbiol Spectr. 2015 Apr;3(2):MDNA3-0042-2014. doi: 10.1128/microbiolspec.MDNA3-0042-2014. Microbiol Spectr. 2015. PMID: 26104705 Review.
Cited by
- Sleeping Beauty Transposon Insertions into Nucleolar DNA by an Engineered Transposase Localized in the Nucleolus.
Kovač A, Miskey C, Ivics Z. Kovač A, et al. Int J Mol Sci. 2023 Oct 7;24(19):14978. doi: 10.3390/ijms241914978. Int J Mol Sci. 2023. PMID: 37834425 Free PMC article. - Current strategies employed in the manipulation of gene expression for clinical purposes.
Tsai HC, Pietrobon V, Peng M, Wang S, Zhao L, Marincola FM, Cai Q. Tsai HC, et al. J Transl Med. 2022 Nov 18;20(1):535. doi: 10.1186/s12967-022-03747-3. J Transl Med. 2022. PMID: 36401279 Free PMC article. Review. - Episomes and Transposases-Utilities to Maintain Transgene Expression from Nonviral Vectors.
Kreppel F, Hagedorn C. Kreppel F, et al. Genes (Basel). 2022 Oct 16;13(10):1872. doi: 10.3390/genes13101872. Genes (Basel). 2022. PMID: 36292757 Free PMC article. Review. - Jumping Ahead with Sleeping Beauty: Mechanistic Insights into Cut-and-Paste Transposition.
Ochmann MT, Ivics Z. Ochmann MT, et al. Viruses. 2021 Jan 8;13(1):76. doi: 10.3390/v13010076. Viruses. 2021. PMID: 33429848 Free PMC article. Review. - In vivo functional screening for systems-level integrative cancer genomics.
Weber J, Braun CJ, Saur D, Rad R. Weber J, et al. Nat Rev Cancer. 2020 Oct;20(10):573-593. doi: 10.1038/s41568-020-0275-9. Epub 2020 Jul 7. Nat Rev Cancer. 2020. PMID: 32636489 Review.
References
- Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, Thrasher AJ, Wulffraat N, Sorensen R, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 2002;346:1185–1193. - PubMed
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. - PubMed
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. - PubMed
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. - PubMed
- Katz RA, Merkel G, Skalka AM. Targeting of retroviral integrase by fusion to a heterologous DNA binding domain: in vitro activities and incorporation of a fusion protein into viral particles. Virology. 1996;217:178–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources