The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells - PubMed (original) (raw)
. 2007 Mar 1;21(5):525-30.
doi: 10.1101/gad.415507.
Daniela Kleine-Kohlbrecher, Nikolaj Dietrich, Diego Pasini, Gaetano Gargiulo, Chantal Beekman, Kim Theilgaard-Mönch, Saverio Minucci, Bo T Porse, Jean-Christophe Marine, Klaus H Hansen, Kristian Helin
Affiliations
- PMID: 17344414
- PMCID: PMC1820894
- DOI: 10.1101/gad.415507
The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells
Adrian P Bracken et al. Genes Dev. 2007.
Erratum in
- Corrigendum: The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells.
Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Mönch K, Minucci S, Porse BT, Marine JC, Hansen KH, Helin K. Bracken AP, et al. Genes Dev. 2023 Sep 1;37(17-18):861. doi: 10.1101/gad.351178.123. Genes Dev. 2023. PMID: 37852794 Free PMC article. No abstract available.
Abstract
The p16INK4A and p14ARF proteins, encoded by the INK4A-ARF locus, are key regulators of cellular senescence, yet the mechanisms triggering their up-regulation are not well understood. Here, we show that the ability of the oncogene BMI1 to repress the INK4A-ARF locus requires its direct association and is dependent on the continued presence of the EZH2-containing Polycomb-Repressive Complex 2 (PRC2) complex. Significantly, EZH2 is down-regulated in stressed and senescing populations of cells, coinciding with decreased levels of associated H3K27me3, displacement of BMI1, and activation of transcription. These results provide a model for how the INK4A-ARF locus is activated and how Polycombs contribute to cancer.
Figures
Figure 1.
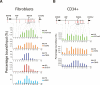
PcG proteins bind throughout the INK4A-ARF locus. (A) ChIP analysis of the INK4B and INK4A-ARF loci in TIG3-TERT HEFs. The precipitated DNA was amplified by real-time qPCR using primers specific for the regions indicated by red bars in the top of the panel. Enrichments are presented as percentages of total input. (B) ChIP analysis of the INK4B and INK4A-ARF loci in CD34-positive BM cells.
Figure 2.
Ectopically expressed BMI1 is recruited to the INK4A-ARF locus. (A) Western blot analysis of LZRS- and LZRSBMI1-infected TIG3-TERT HEFs. (B) qPCR analysis of the mRNA levels of ARF and INK4A in cells from A. (C) ChIP analysis of BMI1, CBX8, and RNA polymerase II enrichments in LZRS- and LZRSBMI1-infected TIG3-TERT HEFs. (D) ChIP analysis at the human “peak” PcG-binding region (primer set 7 in C) and the ARF promoter in LZRS- and LZRSBMI1-infected TIG3-TERT HEFs.
Figure 3.
PcGs and H3K27me3 are lost from the Ink4a-Arf locus in cells undergoing senescence. (A) Western blots of cell lysates prepared from MEFs at increasing passage numbers were probed with the indicated antibodies. (B) qPCR analysis of the indicated genes on mRNA prepared from the cells shown in A. (C) Representation of the mouse Ink4a-Arf gene locus. Amplified regions in D are indicated as red bars. (D) ChIP analysis using the indicated antibodies and the cells shown in A on the Ink4a-Arf locus.
Figure 4.
Targeted depletion of PRC2 displaces BMI1 from the INK4A-ARF locus, increases the expression of INK4A, and leads to senescence. (A) Western blot analysis of cell lysates prepared from TIG3-TERT HEFs infected with pRS, pRSSUZ12, or pRSEZH2, 7 d after selection with puromycin. (B) qPCR analysis of INK4A and ARF mRNA levels from cells from A. (C) ChIP analysis of PcGs and H3K27me3 enrichments on the INK4A and ARF promoters in pRS- and pRSSUZ12-infected cells. (D) β-galactosidase staining to detect senescent cells in TIG3-TERT cells infected with pRS, pRSSUZ12, pRSEZH2, or pRSBMI1 constructs.
Figure 5.
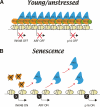
Model for how PcG proteins regulate cellular senescence in vivo. (A) In young proliferating normal diploid cells, the PRC2 complex is highly abundant and maintains the levels of H3K27me3 along the INK4B and INK4A-ARF loci. This in turn ensures the continued association of the BMI1-containing PRC1 complex and repression of the INK4A, INK4B, and ARF genes. (B) In older or stressed cells the levels of EZH2 are down-regulated, leading to the disruption of the PRC2 complex and loss of H3K27me3 along the INK4B and INK4A-ARF loci. Without H3K27me3, the BMI1-containing PRC1 complex is displaced. We propose a model in which cumulative stress leads to the progressive alteration of the chromatin state around the INK4B and INK4A-ARF loci, thereby conferring accessibility to specific transcriptional activators (X, Y and Z).
Similar articles
- Polycomb mediated epigenetic silencing and replication timing at the INK4a/ARF locus during senescence.
Agherbi H, Gaussmann-Wenger A, Verthuy C, Chasson L, Serrano M, Djabali M. Agherbi H, et al. PLoS One. 2009 May 20;4(5):e5622. doi: 10.1371/journal.pone.0005622. PLoS One. 2009. PMID: 19462008 Free PMC article. - A novel zinc finger protein Zfp277 mediates transcriptional repression of the Ink4a/arf locus through polycomb repressive complex 1.
Negishi M, Saraya A, Mochizuki S, Helin K, Koseki H, Iwama A. Negishi M, et al. PLoS One. 2010 Aug 24;5(8):e12373. doi: 10.1371/journal.pone.0012373. PLoS One. 2010. PMID: 20808772 Free PMC article. - Ndy1/KDM2B immortalizes mouse embryonic fibroblasts by repressing the Ink4a/Arf locus.
Tzatsos A, Pfau R, Kampranis SC, Tsichlis PN. Tzatsos A, et al. Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2641-6. doi: 10.1073/pnas.0813139106. Epub 2009 Feb 6. Proc Natl Acad Sci U S A. 2009. PMID: 19202064 Free PMC article. - Long noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression.
Aguilo F, Zhou MM, Walsh MJ. Aguilo F, et al. Cancer Res. 2011 Aug 15;71(16):5365-9. doi: 10.1158/0008-5472.CAN-10-4379. Epub 2011 Aug 9. Cancer Res. 2011. PMID: 21828241 Free PMC article. Review. - Bmi1, stem cells, and senescence regulation.
Park IK, Morrison SJ, Clarke MF. Park IK, et al. J Clin Invest. 2004 Jan;113(2):175-9. doi: 10.1172/JCI20800. J Clin Invest. 2004. PMID: 14722607 Free PMC article. Review.
Cited by
- AKT and EZH2 inhibitors kill TNBCs by hijacking mechanisms of involution.
Schade AE, Perurena N, Yang Y, Rodriguez CL, Krishnan A, Gardner A, Loi P, Xu Y, Nguyen VTM, Mastellone GM, Pilla NF, Watanabe M, Ota K, Davis RA, Mattioli K, Xiang D, Zoeller JJ, Lin JR, Morganti S, Garrido-Castro AC, Tolaney SM, Li Z, Barbie DA, Sorger PK, Helin K, Santagata S, Knott SRV, Cichowski K. Schade AE, et al. Nature. 2024 Oct 9. doi: 10.1038/s41586-024-08031-6. Online ahead of print. Nature. 2024. PMID: 39385030 - PRC1 Protein Subcomplexes Architecture: Focus on the Interplay between Distinct PCGF Subunits in Protein Interaction Networks.
Munawar N, Wynne K, Oliviero G. Munawar N, et al. Int J Mol Sci. 2024 Sep 11;25(18):9809. doi: 10.3390/ijms25189809. Int J Mol Sci. 2024. PMID: 39337298 Free PMC article. - Systematic transcriptomic analysis and temporal modelling of human fibroblast senescence.
Scanlan RL, Pease L, O'Keefe H, Martinez-Guimera A, Rasmussen L, Wordsworth J, Shanley D. Scanlan RL, et al. Front Aging. 2024 Aug 29;5:1448543. doi: 10.3389/fragi.2024.1448543. eCollection 2024. Front Aging. 2024. PMID: 39267611 Free PMC article. - EZH2 represses mesenchymal genes and upholds the epithelial state of breast carcinoma cells.
Gallardo A, López-Onieva L, Belmonte-Reche E, Fernández-Rengel I, Serrano-Prados A, Molina A, Sánchez-Pozo A, Landeira D. Gallardo A, et al. Cell Death Dis. 2024 Aug 22;15(8):609. doi: 10.1038/s41419-024-07011-y. Cell Death Dis. 2024. PMID: 39174513 Free PMC article. - Lysine methylation modifications in tumor immunomodulation and immunotherapy: regulatory mechanisms and perspectives.
Luo Y, Lu J, Lei Z, Zhu H, Rao D, Wang T, Fu C, Zhang Z, Xia L, Huang W. Luo Y, et al. Biomark Res. 2024 Jul 30;12(1):74. doi: 10.1186/s40364-024-00621-w. Biomark Res. 2024. PMID: 39080807 Free PMC article. Review.
References
- Alcorta D.A., Xiong Y., Phelps D., Hannon G., Beach D., Barrett J.C., Xiong Y., Phelps D., Hannon G., Beach D., Barrett J.C., Phelps D., Hannon G., Beach D., Barrett J.C., Hannon G., Beach D., Barrett J.C., Beach D., Barrett J.C., Barrett J.C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. 1996;93:13742–13747. - PMC - PubMed
- Boyer L.A., Plath K., Zeitlinger J., Brambrink T., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K., Plath K., Zeitlinger J., Brambrink T., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K., Zeitlinger J., Brambrink T., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K., Brambrink T., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K., Levine S.S., Wernig M., Tajonar A., Ray M.K., Wernig M., Tajonar A., Ray M.K., Tajonar A., Ray M.K., Ray M.K., et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. - PubMed
- Bracken A.P., Pasini D., Capra M., Prosperini E., Colli E., Helin K., Pasini D., Capra M., Prosperini E., Colli E., Helin K., Capra M., Prosperini E., Colli E., Helin K., Prosperini E., Colli E., Helin K., Colli E., Helin K., Helin K. EZH2 is downstream of the pRB–E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–5335. - PMC - PubMed
- Bracken A.P., Dietrich N., Pasini D., Hansen K.H., Helin K., Dietrich N., Pasini D., Hansen K.H., Helin K., Pasini D., Hansen K.H., Helin K., Hansen K.H., Helin K., Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes & Dev. 2006;20:1123–1136. - PMC - PubMed
- Bruggeman S.W., Valk-Lingbeek M.E., van der Stoop P.P., Jacobs J.J., Kieboom K., Tanger E., Hulsman D., Leung C., Arsenijevic Y., Marino S., Valk-Lingbeek M.E., van der Stoop P.P., Jacobs J.J., Kieboom K., Tanger E., Hulsman D., Leung C., Arsenijevic Y., Marino S., van der Stoop P.P., Jacobs J.J., Kieboom K., Tanger E., Hulsman D., Leung C., Arsenijevic Y., Marino S., Jacobs J.J., Kieboom K., Tanger E., Hulsman D., Leung C., Arsenijevic Y., Marino S., Kieboom K., Tanger E., Hulsman D., Leung C., Arsenijevic Y., Marino S., Tanger E., Hulsman D., Leung C., Arsenijevic Y., Marino S., Hulsman D., Leung C., Arsenijevic Y., Marino S., Leung C., Arsenijevic Y., Marino S., Arsenijevic Y., Marino S., Marino S., et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes & Dev. 2005;19:1438–1443. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases