Multi-site studies of acoustic startle and prepulse inhibition in humans: initial experience and methodological considerations based on studies by the Consortium on the Genetics of Schizophrenia - PubMed (original) (raw)
Multicenter Study
. 2007 May;92(1-3):237-51.
doi: 10.1016/j.schres.2007.01.012. Epub 2007 Mar 8.
Joyce Sprock, Gregory A Light, Kristin Cadenhead, Monica E Calkins, Dorcas J Dobie, Robert Freedman, Michael F Green, Tiffany A Greenwood, Raquel E Gur, Jim Mintz, Ann Olincy, Keith H Nuechterlein, Allen D Radant, Nicholas J Schork, Larry J Seidman, Larry J Siever, Jeremy M Silverman, William S Stone, Debbie W Tsuang, Ming T Tsuang, Bruce I Turetsky, David L Braff
Affiliations
- PMID: 17346930
- PMCID: PMC2039885
- DOI: 10.1016/j.schres.2007.01.012
Multicenter Study
Multi-site studies of acoustic startle and prepulse inhibition in humans: initial experience and methodological considerations based on studies by the Consortium on the Genetics of Schizophrenia
Neal R Swerdlow et al. Schizophr Res. 2007 May.
Abstract
Background: Startle and its inhibition by weak lead stimuli ("prepulse inhibition": PPI) are studied to understand the neurobiology of information processing in patients and community comparison subjects (CCS). PPI has a strong genetic basis in infrahumans, and there is evidence for its heritability, stability and reliability in humans. PPI has gained increasing use as an endophenotype to identify vulnerability genes for brain disorders, including schizophrenia. Genetic studies now often employ multiple, geographically dispersed test sites to accommodate the need for large and complex study samples. Here, we assessed the feasibility of using PPI in multi-site studies.
Methods: Within a 7-site investigation with multiple measures, the Consortium on the Genetics of Schizophrenia conducted a methodological study of acoustic startle and PPI in CCS. Methods were manualized, videotaped and standardized across sites with intensive in-person training sessions. Equipment was acquired and programmed at the "PPI site" (UCSD), and stringent quality assurance (QA) procedures were used. Testing was completed on 196 CCS over 2.5 years, with 5 primary startle dependent measures: eyeblink startle magnitude, habituation, peak latency, latency facilitation and PPI.
Results: Analyses identified significant variability across sites in some but not all primary measures, and determined factors both within the testing process and subject characteristics that influenced a number of test measures. QA procedures also identified non-standardized practices with respect to testing methods and procedural "drift", which may be particularly relevant to multi-site studies using these measures.
Conclusion: With thorough oversight and QA procedures, measures of acoustic startle PPI can be acquired reliably across multiple testing sites. Nonetheless, even among sites with substantial expertise in utilizing psychophysiological measures, multi-site studies using startle and PPI as dependent measures require careful attention to methodological procedures.
Figures
Figure 1
Consortium on the Genetics of Schizophrenia (COGS) structure and interactions. Figure from Calkins et al. (2006), with permission of author. Data were uploaded to a central Data Core website, managed by the UCLA site. Files were accepted only if they met specific size and format criteria. Individual data files were downloaded by the QA site (UCSD), blind to diagnosis and other demographic variables.
Figure 2
Schematic representation of startle trial types (top) and session design (below). The startle test session included 74 active and 18 blank “no-stim” trials (interspersed throughout the session), and lasted 23.5 min, beginning with a 5-min acclimation period with 70-dB(A) SPL white noise that continued throughout the session. Startle stimuli were 40-msec 115-dB(A) SPL noise bursts (near-instantaneous rise time, est. 1 ms). Prepulses were 20 ms noise bursts 15-dB above a 70-dB(A) SPL white noise background, with prepulse onset 30, 60 or 120 ms prior to pulse onset; using slightly more intense 16 dB prepulses with this startle system, prepulse-associated EMG activity is <0.5 % of startle stimulus-induced levels. Five startle stimuli were presented at the beginning (Block 1) and end of the session (Block 4) to assess habituation. In Blocks 2-3, pulse alone and each of the 3 prepulse trial-types was pseudo-randomly intermixed (9 trials per condition per blocks; inter-trial intervals 11-19 s (mean=15 s)). In 18 blank “no-stim” trials, data were recorded without stimulus presentation, to assess basal EMG activity. Subjects were excluded for mean block 2 startle magnitude <10 startle units; this threshold was established to identify the lowest 10% of a normal distribution of startle magnitude values from >200 adult CCS tested at UCSD, using the present stimulus parameters. Applied to the present sample, it identified the lowest 11.7%.
Figure 3
Mean (SEM) values (A) and individual distributions (B) for startle magnitude during session blocks 2 and 3, when PPI is measured, at the 7 COGS test sites (A-G). 1 startle unit = 1.31 μV. The focus of this study was the comparison of startle measures across test sites, and thus separate analyses of each startle variable at each site are not described. Nonetheless, of the primary startle measures that involve a within-subject comparison -- habituation (startle magnitude in block 1 vs. 4), prepulse facilitation of latency (reduction of onset and peak latency on prepulse trials compared to pulse alone trials), and PPI (reduction of startle magnitude on prepulse + pulse trials compared to pulse alone trials) -- all are highly significant at each individual site.
Figure 4
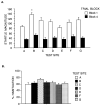
Startle habituation, shown as mean (SEM) startle magnitude during blocks 1 and 4 (A), and as a percent score (B), across the 7 COGS sites. 1 startle unit = 1.31 μV. * = p < 0.05, B vs. A, E and F
Figure 5
Peak startle latency (ms (SEM)), on PA trials (0 ms prepulse intervals), and 30, 60 and 120 ms prepulse+pulse trials, across the 7 COGS sites. Inset shows distinct patterns of peak reflex latency modulation in women vs. men. Despite the lack of significant site × trial type interaction for peak latency, inspection of the data revealed some inter-site variability in the patterns of latency facilitation across the different prepulse intervals. For example, peak latency for 120 ms prepulse trials was significantly slower than for 30 and 60 ms trials for some sites (A,B,C,G) but not others (D,F), and fastest latencies were detected with 30 ms prepulse trials at some sites, and with 60 ms prepulse trials at others. Neither hearing threshold nor electrode impedance correlated significantly with reflex latency on PA or any prepulse trials (all r's<0.13). No significant relationships were detected between reflex latency and either WRAT scores or ethnicity. Ambient noise levels also did not correlate significantly with mean peak latency for each site on PA (rs= -0.03, p=0.93) or on any prepulse trials (rs's= -0.24 - (-0.01); p's=0.54 - 0.97). ANOVA across all sites revealed a significant interaction of gender × trial type (F=3.33, df 3,447, p<0.02). As seen in the inset, this interaction is reflected in patterns of reflex latency across sites: sites testing predominantly men (e.g. site F) exhibited latency patterns characteristic of males in the present study (minimal difference in latency across prepulse intervals), and those testing predominantly women (e.g. site A) exhibited latency patterns characteristic of females in the present study (less facilitation with 120 vs. 60 ms prepulse intervals).
Figure 6
A. Mean %PPI (SEM) with 30, 60 and 120 ms prepulse+pulse trials across the 7 COGS sites. B. %PPI in men and women, shown in test orders A and B. * = p < 0.0003, significant effect of test order in men; # = p < 0.015, men > women, test order A, after significant interaction of sex × test order, p < 0.02.
Similar articles
- Heritability of acoustic startle magnitude, prepulse inhibition, and startle latency in schizophrenia and control families.
Hasenkamp W, Epstein MP, Green A, Wilcox L, Boshoven W, Lewison B, Duncan E. Hasenkamp W, et al. Psychiatry Res. 2010 Jul 30;178(2):236-43. doi: 10.1016/j.psychres.2009.11.012. Epub 2010 May 16. Psychiatry Res. 2010. PMID: 20483176 Free PMC article. - Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia.
Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX. Ludewig K, et al. Schizophr Res. 2002 May 1;55(1-2):129-37. doi: 10.1016/s0920-9964(01)00198-0. Schizophr Res. 2002. PMID: 11955972 - Prepulse inhibition of acoustic startle in Japanese patients with chronic schizophrenia.
Kunugi H, Tanaka M, Hori H, Hashimoto R, Saitoh O, Hironaka N. Kunugi H, et al. Neurosci Res. 2007 Sep;59(1):23-8. doi: 10.1016/j.neures.2007.05.006. Epub 2007 May 25. Neurosci Res. 2007. PMID: 17692982 - [The startle reflex in schizophrenia research].
Meincke U, Gouzoulis-Mayfrank E, Sass H. Meincke U, et al. Nervenarzt. 2001 Nov;72(11):844-52. doi: 10.1007/s001150170018. Nervenarzt. 2001. PMID: 11758091 Review. German. - Meta-analysis on the association between genetic polymorphisms and prepulse inhibition of the acoustic startle response.
Quednow BB, Ejebe K, Wagner M, Giakoumaki SG, Bitsios P, Kumari V, Roussos P. Quednow BB, et al. Schizophr Res. 2018 Aug;198:52-59. doi: 10.1016/j.schres.2017.12.011. Epub 2017 Dec 26. Schizophr Res. 2018. PMID: 29287625 Review.
Cited by
- Deficient prepulse inhibition in schizophrenia in a multi-site cohort: Internal replication and extension.
Swerdlow NR, Light GA, Thomas ML, Sprock J, Calkins ME, Green MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Swerdlow NR, et al. Schizophr Res. 2018 Aug;198:6-15. doi: 10.1016/j.schres.2017.05.013. Epub 2017 May 24. Schizophr Res. 2018. PMID: 28549722 Free PMC article. - Between-site reliability of startle prepulse inhibition across two early psychosis consortia.
Cadenhead KS, Addington J, Cannon TD, Cornblatt BA, de la Fuente-Sandoval C, Mathalon DH, Perkins DO, Seidman LJ, Tsuang M, Walker EF, Woods SW, Bachman P, Belger A, Carrión RE, Donkers FC, Duncan E, Johannesen J, León-Ortiz P, Light G, Mondragón A, Niznikiewicz M, Nunag J, Roach BJ, Solís-Vivanco R; North American Prodromal Longitudinal Studies Consortium. Cadenhead KS, et al. Neuroreport. 2013 Aug 7;24(11):626-30. doi: 10.1097/WNR.0b013e3283637845. Neuroreport. 2013. PMID: 23799460 Free PMC article. - Strain differences in the disruption of prepulse inhibition of startle after systemic and intra-accumbens amphetamine administration.
Swerdlow NR, Shoemaker JM, Bongiovanni MJ, Neary AC, Tochen LS, Saint Marie RL. Swerdlow NR, et al. Pharmacol Biochem Behav. 2007 May;87(1):1-10. doi: 10.1016/j.pbb.2007.03.014. Epub 2007 Apr 3. Pharmacol Biochem Behav. 2007. PMID: 17475315 Free PMC article. - The Effects of Attention on the Syllable-Induced Prepulse Inhibition of the Startle Reflex and Cortical EEG Responses against Energetic or Informational Masking in Humans.
Yang X, Liu L, Yang P, Ding Y, Wang C, Li L. Yang X, et al. Brain Sci. 2022 May 18;12(5):660. doi: 10.3390/brainsci12050660. Brain Sci. 2022. PMID: 35625046 Free PMC article. - Comparison of the heritability of schizophrenia and endophenotypes in the COGS-1 family study.
Light G, Greenwood TA, Swerdlow NR, Calkins ME, Freedman R, Green MF, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Light G, et al. Schizophr Bull. 2014 Nov;40(6):1404-11. doi: 10.1093/schbul/sbu064. Epub 2014 Jun 5. Schizophr Bull. 2014. PMID: 24903414 Free PMC article.
References
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) University of Iowa; Iowa City, IA: 1984.
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City: 1984.
- Anokhin AP, Heath AC, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of startle reflex in humans. Neurosci. Lett. 2003;353:45–8. - PubMed
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. - PubMed
- Braff DL, Freedman R. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins; Baltimore: 2002. The importance of endophenotypes in studies of the genetics of schizophrenia; pp. 703–716.
Publication types
MeSH terms
Grants and funding
- R01 MH065571-03/MH/NIMH NIH HHS/United States
- R01 MH043518/MH/NIMH NIH HHS/United States
- K02 MH001436-09/MH/NIMH NIH HHS/United States
- R01 MH042228-19/MH/NIMH NIH HHS/United States
- R01 MH084071/MH/NIMH NIH HHS/United States
- R01 MH042228/MH/NIMH NIH HHS/United States
- R01 MH065571-04/MH/NIMH NIH HHS/United States
- R01 MH065571/MH/NIMH NIH HHS/United States
- R01 MH042228-17A1/MH/NIMH NIH HHS/United States
- K02 MH001436-08/MH/NIMH NIH HHS/United States
- R01 MH065571-02/MH/NIMH NIH HHS/United States
- R01 MH065558/MH/NIMH NIH HHS/United States
- K02 MH001436-10/MH/NIMH NIH HHS/United States
- R01 MH065571-01A1/MH/NIMH NIH HHS/United States
- R01 MH065554/MH/NIMH NIH HHS/United States
- R01 MH065578/MH/NIMH NIH HHS/United States
- R01 MH065562/MH/NIMH NIH HHS/United States
- K02 MH001436-07/MH/NIMH NIH HHS/United States
- R01 MH065588/MH/NIMH NIH HHS/United States
- R01 MH042228-20/MH/NIMH NIH HHS/United States
- R01 MH042228-18/MH/NIMH NIH HHS/United States
- K02 MH001436/MH/NIMH NIH HHS/United States
- R01 MH065707/MH/NIMH NIH HHS/United States
- R37 MH043518/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical