Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha - PubMed (original) (raw)
Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha
Zachary Gerhart-Hines et al. EMBO J. 2007.
Abstract
In mammals, maintenance of energy and nutrient homeostasis during food deprivation is accomplished through an increase in mitochondrial fatty acid oxidation in peripheral tissues. An important component that drives this cellular oxidative process is the transcriptional coactivator PGC-1alpha. Here, we show that fasting induced PGC-1alpha deacetylation in skeletal muscle and that SIRT1 deacetylation of PGC-1alpha is required for activation of mitochondrial fatty acid oxidation genes. Moreover, expression of the acetyltransferase, GCN5, or the SIRT1 inhibitor, nicotinamide, induces PGC-1alpha acetylation and decreases expression of PGC-1alpha target genes in myotubes. Consistent with a switch from glucose to fatty acid oxidation that occurs in nutrient deprivation states, SIRT1 is required for induction and maintenance of fatty acid oxidation in response to low glucose concentrations. Thus, we have identified SIRT1 as a functional regulator of PGC-1alpha that induces a metabolic gene transcription program of mitochondrial fatty acid oxidation. These results have implications for understanding selective nutrient adaptation and how it might impact lifespan or metabolic diseases such as obesity and diabetes.
Figures
Figure 1
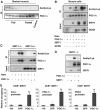
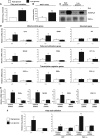
SIRT1 deacetylates PGC-1α in skeletal muscle cells. (A) Fasting-induced PGC-1α deacetylation in skeletal muscle. Mice were either fed or fasted for 16 h and skeletal muscle was used to immunoprecipitate PGC-1α and Western blot was performed to detect PGC-1α levels and acetylation. (B) Nicotinamide and GCN5 induce PGC-1α acetylation. C2C12 myotubes were treated with 5 mM nicotinamide for 12 h and/or infected with the indicated adenoviruses for 48 h. After treatment, cells were harvested and protein cell extracts were used for PGC-1α immunoprecipitation and Western blot analysis using the specified antibodies. (C) PGC-1α is constitutively acetylated in SIRT1−/− MEFs. MEFs were treated with nicotinamide and/or infected with adenoviruses encoding PGC-1α as described in (B). (D) SIRT1 rescues PGC-1α deacetylation in SIRT1−/− MEFs. Adenoviruses expressing PGC-1α and SIRT1 were infected in MEFs as described in (B). (E) SIRT1 is bound to PGC-1α-targeted gene promoters. C2C12 myotubes were infected with adenoviruses encoding either GFP or PGC-1α. Forty-eight hours after infection, cells were harvested and ChIP analysis using SIRT1 antibody was performed as described in Materials and methods. Values in (E) represent the mean of two experiments performed in triplicate. Error bars represent s.e.m. Statistical analyses were performed using Student's _t_-test. *P<0.05 and **P<0.005.
Figure 2
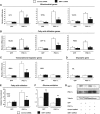
SIRT1 regulates PGC-1α-mediated mitochondrial and fatty acid metabolism in C2C12 skeletal muscle cells. (A) SIRT1 knockdown decreases PGC-1α-induced mitochondrial, (B) fatty acid utilization, (C) ERRα, but not (D) pyruvate kinase gene expression. C2C12 myotubes were infected for 3 days with the indicated adenoviruses. After harvesting, RNA was extracted and used for measuring indicated gene expression using quantitative RT–PCR analysis. (E) PGC-1α-induced oxidation rates of fatty acids are reduced by SIRT1 knockdown. C2C12 myotubes were treated as in (A). Oleic acid oxidation rates were measured as described in Materials and methods. (F) PGC-1α-decreased oxidation rates of glucose are prevented by SIRT1 knockdown. Glucose oxidation rates were measured in C2C12 myotubes as described in Materials and methods. (G) Knockdown of SIRT1 in C2C12 myotubes. Cells were infected with the different adenoviruses as in (A–F). Protein cell extracts were used for Western blot analysis that was performed with the indicated antibodies. Values represent the mean of 2–3 experiments performed in duplicate. Error bars represent s.e.m. Statistical analyses were performed using Student's _t_-test. *P<0.05 and **P<0.005, shRNA control versus shRNA SIRT1.
Figure 3
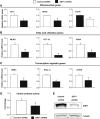
SIRT1 regulates mitochondrial and fatty acid oxidation genes in primary skeletal muscle cells. (A) SIRT1 knockdown decreases mitochondrial genes, (B) fatty acid utilization genes and (C) transcriptional regulator gene expresson in primary myotubes. Mouse primary myotubes were transduced with adenovirus expressing either SIRT1 shRNA or control shRNA for 71 h. Total RNA was extracted and used for measuring indicated gene expression using quantitative RT–PCR. (D) SIRT1 knockdown decreases citrate synthase enzymatic activity in primary myotubes. (E) Knockdown of SIRT1 in primary skeletal myotubes. Cells were infected with the indicated adenoviruses and protein cell extracts were used to perform Western blot analysis for SIRT1 and tubulin as a control. Values represent the mean of 2–3 experiments performed in duplicate. Error bars represent s.e.m. Statistical analyses were performed using Student's _t_-test. *P<0.05. shRNA control versus shRNA SIRT1.
Figure 4
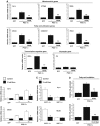
SIRT1 regulates mitochondrial and fatty acid metabolism in MEFs. Decreased mitochondrial (A), fatty acid utilization (B) and mitochondrial transcriptional regulator (C) gene expression in SIRT1−/− MEFs. MEFs were cultured until confluence, harvested and RNA was extracted to measure mitochondrial genes by quantitative RT–PCR. (D) Ectopic expression of SIRT1 rescues mitochondrial and fatty acid utilization gene expression. SIRT1−/− MEFs were infected with different amounts of adenoviruses encoding for SIRT1. After 3 days of infection, total RNA was isolated and indicated gene expressions were measured by quantitative RT–PCR. (E) Decreased rates of fatty acid oxidation in SIRT1−/− MEFs. Cells were treated as described in (A), but were used to measure oleic acid oxidation rates as described in Materials and methods. Values represent the mean of 2–3 experiments performed in duplicate. Error bars represent s.e.m. Statistical analyses were performed using Student's _t_-test. *P<0.05 and **P<0.005.
Figure 5
GCN5 regulates mitochondrial and fatty acid metabolism in skeletal muscle cells. (A) GCN5 downregulates PGC-1α-induced mitochondrial gene expression. C2C12 myotubes were infected for 3 days with the indicated adenoviruses. Total RNA was extracted and analyzed for mitochondrial, fatty acid utilization and transcriptional regulator gene expression using quantitative RT–PCR analysis. (B) Nicotinamide downregulates PGC-1α-induced mitochondrial and fatty acid oxidation gene expression in C2C12 myotubes and (C) MEFs. C2C12 cells were infected with the indicated adenoviruses. At day 3 after infection, cells were treated for 12 h with 10 mM nicotinamide. Confluent MEFs were also treated for 12 h with nicotinamide and harvested for RNA analysis. (D) GCN5 and nicotinamide prevented PGC-1α-increased fatty acid oxidation rates. Cells were treated as in (A–C), but oleic acid oxidation assays were performed as described in Materials and methods. Values represent the mean of 2–3 experiments performed in duplicate. Error bars represent s.e.m. Statistical analyses were performed using Student's _t_-test. *P<0.05 and **P<0.005.
Figure 6
SIRT1 is required for the switch to fatty acid oxidation in response to low glucose concentrations. (A) Low glucose concentration induced increase in rates of fatty acid oxidation and (B) increased in cellular NAD+ levels. C2C12 myotubes were treated with 25 mM (open bars) or 5 mM (filled bars) glucose for 12 h. Palmitic acid oxidation was measured and in another set of experiments, deproteinized cell extracts were used to determine NAD+ levels, as described in Materials and methods. (C) Low glucose concentration induces deacetylation of PGC-1α. C2C12 myotubes were infected with PGC-1α adenoviruses and treated for 12 h with 25 and 5 mM of glucose with or without nicotinamide (10 mM). (D) SIRT1 is required to increase mitochondrial and fatty acid utilization genes in response to low glucose in MEFs and (E) C2C12 myotubes. C2C12 cells were infected with the adenoviruses encoding PGC-1α and shRNA control or shRNA SIRT1. Twelve hours before harvesting, MEFs or C2C12 cells were treated with different concentrations of glucose and indicated gene expression was analyzed. (F) SIRT1 is required for induction of mitochondrial fatty acid oxidation in response to low glucose. C2C12 myotubes and MEFs were treated as in (D). Oxidation rates of oleic acid were analyzed as described in Materials and methods. Values represent the mean of 2–3 experiments performed in duplicate. Error bars represent s.e.m. Statistical analyses were performed using Student's _t_-test. *P<0.05 and **P<0.005.
Figure 7
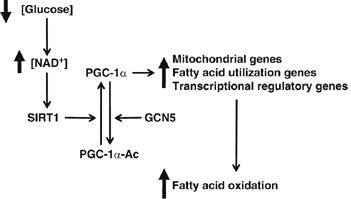
Model of glucose-dependent control of fatty acid oxidation through SIRT1/PGC-1α. Decreases in glucose elevate intracellular levels of NAD+ that will activate SIRT1deacetylase enzymatic activity. SIRT1 interacts and deacetylates PGC-1α, which induces an upregulation of genes linked to mitochondrial function and fatty acid utilization. Finally, this change in PGC-1_α_-targeted gene expression results in the activation of complete oxidation of fatty acids. See text for further details.
Similar articles
- Interactions between the consumption of a high-fat diet and fasting in the regulation of fatty acid oxidation enzyme gene expression: an evaluation of potential mechanisms.
Frier BC, Jacobs RL, Wright DC. Frier BC, et al. Am J Physiol Regul Integr Comp Physiol. 2011 Feb;300(2):R212-21. doi: 10.1152/ajpregu.00367.2010. Epub 2010 Nov 17. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21084676 - Activation of SIRT1 by L-serine increases fatty acid oxidation and reverses insulin resistance in C2C12 myotubes.
Sim WC, Kim DG, Lee W, Sim H, Choi YJ, Lee BH. Sim WC, et al. Cell Biol Toxicol. 2019 Oct;35(5):457-470. doi: 10.1007/s10565-019-09463-x. Epub 2019 Feb 5. Cell Biol Toxicol. 2019. PMID: 30721374 - Dietary enrichment with fish oil prevents high fat-induced metabolic dysfunction in skeletal muscle in mice.
Philp LK, Heilbronn LK, Janovska A, Wittert GA. Philp LK, et al. PLoS One. 2015 Feb 6;10(2):e0117494. doi: 10.1371/journal.pone.0117494. eCollection 2015. PLoS One. 2015. PMID: 25658742 Free PMC article. - Exercise testing in metabolic myopathies.
Tarnopolsky M. Tarnopolsky M. Phys Med Rehabil Clin N Am. 2012 Feb;23(1):173-86, xii. doi: 10.1016/j.pmr.2011.11.011. Epub 2011 Dec 11. Phys Med Rehabil Clin N Am. 2012. PMID: 22239882 Review. - Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis.
Gurd BJ. Gurd BJ. Appl Physiol Nutr Metab. 2011 Oct;36(5):589-97. doi: 10.1139/h11-070. Epub 2011 Sep 2. Appl Physiol Nutr Metab. 2011. PMID: 21888529 Review.
Cited by
- Effects of Exhaustive Aerobic Exercise on Tryptophan-Kynurenine Metabolism in Trained Athletes.
Strasser B, Geiger D, Schauer M, Gatterer H, Burtscher M, Fuchs D. Strasser B, et al. PLoS One. 2016 Apr 28;11(4):e0153617. doi: 10.1371/journal.pone.0153617. eCollection 2016. PLoS One. 2016. PMID: 27124720 Free PMC article. - Targeting Epigenetic Regulators with HDAC and BET Inhibitors to Modulate Muscle Wasting.
Nevi L, Pöllänen N, Penna F, Caretti G. Nevi L, et al. Int J Mol Sci. 2023 Nov 16;24(22):16404. doi: 10.3390/ijms242216404. Int J Mol Sci. 2023. PMID: 38003594 Free PMC article. Review. - Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart.
Aubert G, Vega RB, Kelly DP. Aubert G, et al. Biochim Biophys Acta. 2013 Apr;1833(4):840-7. doi: 10.1016/j.bbamcr.2012.08.015. Epub 2012 Aug 31. Biochim Biophys Acta. 2013. PMID: 22964268 Free PMC article. Review. - Nature's Timepiece-Molecular Coordination of Metabolism and Its Impact on Aging.
Bednářová A, Kodrík D, Krishnan N. Bednářová A, et al. Int J Mol Sci. 2013 Jan 31;14(2):3026-49. doi: 10.3390/ijms14023026. Int J Mol Sci. 2013. PMID: 23434656 Free PMC article. - Increasing dietary fat elicits similar changes in fat oxidation and markers of muscle oxidative capacity in lean and obese humans.
Bergouignan A, Gozansky WS, Barry DW, Leitner W, MacLean PS, Hill JO, Draznin B, Melanson EL. Bergouignan A, et al. PLoS One. 2012;7(1):e30164. doi: 10.1371/journal.pone.0030164. Epub 2012 Jan 12. PLoS One. 2012. PMID: 22253914 Free PMC article. Clinical Trial.
References
- Bordone L, Guarente L (2005) Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6: 298–305 - PubMed
- Chua KF, Mostoslavsky R, Lombard DB, Pang WW, Saito S, Franco S, Kaushal D, Cheng HL, Fischer MR, Stokes N, Murphy MM, Appella E, Alt FW (2005) Mammalian SIRT1 limits replicative lifespan in response to chronic genotoxic stress. Cell Metab 2: 67–76 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases