Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) - PubMed (original) (raw)
Review
Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here)
James W Putney Jr. Cell Calcium. 2007 Aug.
Abstract
Activation of phospholipase C by G-protein-coupled receptors results in release of intracellular Ca(2+) and activation of Ca(2+) channels in the plasma membrane. The intracellular release of Ca(2+) is signaled by the second messenger, inositol 1,4,5-trisphosphate. Ca(2+) entry involves signaling from depleted intracellular stores to plasma membrane Ca(2+) channels, a process referred to as capacitative calcium entry or store-operated calcium entry. The electrophysiological current associated with capacitative calcium entry is the calcium-release-activated calcium current, or I(crac). In the 20 years since the inception of the concept of capacitative calcium entry, a variety of activation mechanisms have been proposed, and there has been considerable interest in the possibility of transient receptor potential channels functioning as store-operated channels. However, in the past 2 years, two major players in both the signaling and permeation mechanisms for store-operated channels have been discovered: Stim1 (and possibly Stim2) and the Orai proteins. Activation of store-operated channels involves an endoplasmic reticulum Ca(2+) sensor called Stim1. Stim1 acts by redistributing within a small component of the endoplasmic reticulum, approaching the plasma membrane, but does not appear to translocate into the plasma membrane. Stim1, either directly or indirectly, signals to plasma membrane Orai proteins which constitute pore-forming subunits of store-operated channels.
Figures
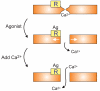
Figure 1. Primitive version of capacitative calcium entry
The intracellular Ca2+ store was envisioned as bound to the plasma membrane, occluding the Ca2+ channel. release of the bound Ca2+ was associated with gating of the Ca2+ channel. Redrawn from [3] with permission.
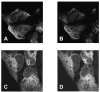
Figure 2. Stim1 rearranges following Ca2+ store depletion, but the endoplasmic reticulum does not
A and B: Thapsigargin causes rearrangement of YFP-Stim1 from a fibrillar or tubular arrangement into punctae. From [49] with permission. C and D: Endoplasmic reticulum-targeted GFP does not redistribute or rearrange following Ca2+ store depletion. From [53] with permission.
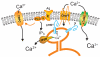
Figure 3. Current understanding of the roles of Stim1 and Orais
Agonist activation of a plasma membrane receptor (R) results in formation of IP3, which activates the IP3 receptor (IP3R) causing discharge of store Ca2+ from a subcompartment of the endoplasmic reticulum. Within this subcompartment, Ca2+ binds reversibly to an EF hand motif in Stim1; depletion of Ca2+ results in Stim1 without Ca2+ bound, which causes Stim1 to redistribute within the endoplasmic reticulum to areas near Orai within the plasma membrane. Stim1 then activates Ca2+-selective Orai channels; the mechanism whereby this activation is accomplished is unknown. Stim1 is also present in the plasma membrane, although its function there is unclear.
Similar articles
- New molecular players in capacitative Ca2+ entry.
Putney JW Jr. Putney JW Jr. J Cell Sci. 2007 Jun 15;120(Pt 12):1959-65. doi: 10.1242/jcs.03462. Epub 2007 May 3. J Cell Sci. 2007. PMID: 17478524 Free PMC article. - Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review. - Pharmacology of Store-Operated Calcium Entry Channels.
Bird GS, Putney JW Jr. Bird GS, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 16. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 16. PMID: 30299647 Free Books & Documents. Review. - Calcium influx mechanisms underlying calcium oscillations in rat hepatocytes.
Jones BF, Boyles RR, Hwang SY, Bird GS, Putney JW. Jones BF, et al. Hepatology. 2008 Oct;48(4):1273-81. doi: 10.1002/hep.22461. Hepatology. 2008. PMID: 18802964 Free PMC article. - Alternative forms of the store-operated calcium entry mediators, STIM1 and Orai1.
Putney JW. Putney JW. Curr Top Membr. 2013;71:109-23. doi: 10.1016/B978-0-12-407870-3.00005-6. Curr Top Membr. 2013. PMID: 23890113 Review.
Cited by
- Alkaline pH Promotes NADPH Oxidase-Independent Neutrophil Extracellular Trap Formation: A Matter of Mitochondrial Reactive Oxygen Species Generation and Citrullination and Cleavage of Histone.
Naffah de Souza C, Breda LCD, Khan MA, de Almeida SR, Câmara NOS, Sweezey N, Palaniyar N. Naffah de Souza C, et al. Front Immunol. 2018 Jan 9;8:1849. doi: 10.3389/fimmu.2017.01849. eCollection 2017. Front Immunol. 2018. PMID: 29375550 Free PMC article. - Calcium-sensing receptor modulates extracellular Ca(2+) entry via TRPC-encoded receptor-operated channels in human aortic smooth muscle cells.
Chow JY, Estrema C, Orneles T, Dong X, Barrett KE, Dong H. Chow JY, et al. Am J Physiol Cell Physiol. 2011 Aug;301(2):C461-8. doi: 10.1152/ajpcell.00389.2010. Epub 2011 May 11. Am J Physiol Cell Physiol. 2011. PMID: 21562303 Free PMC article. - FAD mutations in amyloid precursor protein do not directly perturb intracellular calcium homeostasis.
Stieren E, Werchan WP, El Ayadi A, Li F, Boehning D. Stieren E, et al. PLoS One. 2010 Aug 5;5(8):e11992. doi: 10.1371/journal.pone.0011992. PLoS One. 2010. PMID: 20700539 Free PMC article. - Reduction in TRPC4 expression specifically attenuates G-protein coupled receptor-stimulated increases in intracellular calcium in human myometrial cells.
Ulloa A, Gonzales AL, Zhong M, Kim YS, Cantlon J, Clay C, Ku CY, Earley S, Sanborn BM. Ulloa A, et al. Cell Calcium. 2009 Jul;46(1):73-84. doi: 10.1016/j.ceca.2009.05.003. Epub 2009 Jun 11. Cell Calcium. 2009. PMID: 19523685 Free PMC article. - Novel types of Ca2+ release channels participate in the secretory cycle of Paramecium cells.
Ladenburger EM, Sehring IM, Korn I, Plattner H. Ladenburger EM, et al. Mol Cell Biol. 2009 Jul;29(13):3605-22. doi: 10.1128/MCB.01592-08. Epub 2009 Apr 20. Mol Cell Biol. 2009. PMID: 19380481 Free PMC article.
References
- Putney JW., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. - PubMed
- Putney JW., Jr. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous