Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit - PubMed (original) (raw)
Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit
Michaela Rohrmoser et al. Mol Cell Biol. 2007 May.
Abstract
The PeBoW complex is essential for cell proliferation and maturation of the large ribosomal subunit in mammalian cells. Here we examined the role of PeBoW-specific proteins Pes1, Bop1, and WDR12 in complex assembly and stability, nucleolar transport, and pre-ribosome association. Recombinant expression of the three subunits is sufficient for complex formation. The stability of all three subunits strongly increases upon incorporation into the complex. Only overexpression of Bop1 inhibits cell proliferation and rRNA processing, and its negative effects could be rescued by coexpression of WDR12, but not Pes1. Elevated levels of Bop1 induce Bop1/WDR12 and Bop1/Pes1 subcomplexes. Knockdown of Bop1 abolishes the copurification of Pes1 with WDR12, demonstrating Bop1 as the integral component of the complex. Overexpressed Bop1 substitutes for endogenous Bop1 in PeBoW complex assembly, leading to the instability of endogenous Bop1. Finally, indirect immunofluorescence, cell fractionation, and sucrose gradient centrifugation experiments indicate that transport of Bop1 from the cytoplasm to the nucleolus is Pes1 dependent, while Pes1 can migrate to the nucleolus and bind to preribosomal particles independently of Bop1. We conclude that the assembly and integrity of the PeBoW complex are highly sensitive to changes in Bop1 protein levels.
Figures
FIG. 1.
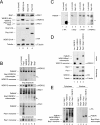
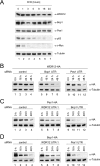
Overexpression of Bop1 inhibits cell proliferation and pre-rRNA processing. (A) Equal numbers of H1299 and TGR-1 cells stably transfected with the indicated constructs were seeded in the presence of 1 μg/ml doxycycline. After 6 days, cells were trypsinized and counted by trypan blue exclusion. The histogram depicts the cell counts relative to that of the mock-treated cell line expressing luciferase. Error bars indicate standard deviations. (B) Expression levels of HA-tagged proteins were determined with the anti-HA antibody 3F10. Equal loading was verified by immunodetection of α-tubulin. (C) The proliferation of stable polyclonal H1299 cell lines expressing the indicated proteins was analyzed as described for panel A. (D) Expression levels of HA-tagged proteins were determined as described for panel B. (E) Diagram of the primary 47S rRNA transcript and the major rRNA intermediates. Positions of the hybridization probes are depicted. ETS, external transcribed spacer. (F) Northern blot analysis of rRNA precursors. Total RNA was extracted from subconfluent H1299 cells expressing the indicated HA-tagged proteins for 24 h. Equal amounts of total RNA were separated by agarose-formaldehyde gel electrophoresis and hybridized with probes specific for ITS-1 and ITS-2 of the rRNA intermediates. As a loading control, blots were incubated with a probe specific for 18S rRNA. (G) Ratios of 47S-45S/18S and 32S/18S rRNAs.
FIG. 2.
(A) Overexpression of Bop1 reduces the endogenous Bop1 protein level. H1299 cells were stably transfected with the indicated constructs. After 6 days, endogenous Pes1, Bop1, and WDR12 protein levels were analyzed by Western blotting. Human-specific anti-Bop1 MAb 6H11 does not recognize recombinant mouse Bop1. Expression levels of HA-tagged proteins were determined with anti-HA antibody 3F10. Equal loading was verified by immunodetection of α-tubulin. (B) Overexpression of Bop1 titrates endogenous WDR12 into a Bop1/WDR12 subcomplex. Total cell lysates of U2OS cells expressing the indicated proteins were separated by native gel electrophoresis after 1 day of expression. Pes1, Bop1, and WDR12 were visualized by immunoblotting. Nonincorporated proteins or complexes containing the respective factor are indicated. (C) Overexpression of Bop1-HA replaces endogenous Bop1 in PeBoW complex formation. Total lysates of U2OS cells expressing luciferase or Bop1-HA were separated by native gel electrophoresis after 6 days of expression. Pes1, Bop1, WDR12, and Bop1-HA were visualized by immunoblotting. An asterisk indicates monomeric Bop1-HA. Double asterisks indicate the Bop1/WDR12 subcomplex. (D) Total lysates of U2OS cells expressing the indicated proteins were separated by native gel electrophoresis. WDR12 were visualized by immunoblotting. Nonincorporated proteins or complexes containing the respective factor are indicated. Expression levels of HA-tagged proteins were determined by Western blot analysis with anti-HA antibody 3F10. Equal loading was verified by immunodetection of α-tubulin. (E) Cytoplasmic and nuclear fractions of H1299 cells expressing the indicated genes were analyzed by native gel electrophoresis as described for panel B. Complexes were visualized by immunostaining with HA-specific antibodies.
FIG. 3.
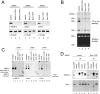
Bop1 is the core factor of the PeBoW complex. (A) U2OS cells were transfected twice with the indicated siRNAs. Endogenous protein levels were analyzed by Western blotting 2 days after the last transfection. α-Tubulin is shown as a loading control. (B) Cells were treated as described for panel A and metabolically labeled with [32P]orthophosphate for 60 min 1 day after the last transfection. Subsequently, cells were incubated for 2 h in regular culture medium. Labeled rRNAs are indicated. Ethidium bromide staining is shown as a loading control. (C) Total cell lysates of U2OS cells transfected twice with the indicated siRNAs were separated by native gel electrophoresis 1 day after the last transfection. Endogenous Pes1, Bop1, and WDR12 were visualized by immunoblotting. Nonincorporated proteins or complexes containing the respective factor are indicated. (D) U2OS cells were transfected at days 0 and 1 with either control or Bop1-specific siRNA. Total cell lysates were subjected to immunoprecipitation with antibodies either against Pes1 (8E9), Bop1 (6H11), WDR12 (1B8), or the isotype control coupled to protein G-Sepharose beads 2 days after the last transfection. Equivalent amounts of total lysates used for immunoprecipitation (IP) were loaded in each lane or 20% thereof for the input. Immunodetection was performed with the anti-Pes1 (8E9) or anti-WDR12 (1B8) antibody, respectively. An asterisk indicates cross-reactivity to immunoglobulin molecules.
FIG. 4.
Specific interdependency of the PeBoW components. (A) H1299 cells were transfected two times with the indicated siRNAs. Effects on the Pes1, Bop1, and WDR12 protein levels were monitored at day 3 after the last transfection by Western blot analysis. (B) A schematic overview of the interdependency of the PeBoW components Pes1, Bop1, and WDR12 is shown. The number of arrows indicates the extent of downregulation.
FIG. 5.
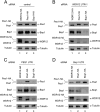
Knockdown-knock-in assay of stable polyclonal H1299 cell lines expressing Pes1-HA, Bop1-HA, or WDR12-HA. Cells were treated with (A) control or (B) WDR12 (3′ UTR)-, (C) Pes1 (3′ UTR)-, or (D) Bop1 (3′ UTR)-specific siRNA. Endogenous Pes1, Bop1, and WDR12 protein levels were analyzed 2 days after the last transfection by Western blotting. Human-specific anti-Bop1 MAb 6H11 does not recognize recombinant mouse Bop1. α-Tubulin is shown as a loading control.
FIG. 6.
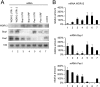
Knockdown of Pes1, Bop1, or WDR12 does not influence the steady-state mRNA levels of the PeBoW components. (A) U2OS cells were transfected twice with the indicated siRNAs. Levels of the mRNAs of Pes1, Bop1, and WDR12 were analyzed 2 days after the last transfection by Northern blot analysis. As a loading control, blots were incubated with a probe specific for 18S rRNA. (B) The histogram depicts quantification of the mRNA levels of Pes1, Bop1, and WDR12 relative to that of a cell line transfected with control siRNA. Error bars indicate standard deviations.
FIG. 7.
Knockdown of Pes1, Bop1, or WDR12 influences the stability of the PeBoW components. (A) U2OS cells were treated with 50 μg/ml cycloheximide (CHX) for the indicated times. The stability of the indicated endogenous proteins was monitored by Western blot analysis. Immunodetection was performed with antibodies against Pes1, Bop1, WDR12, p53 (PAb240), and c-Myc (N-262). Equal loading was verified by immunodetection of α-tubulin. (B to D) H1299 cells stably transfected with the indicated constructs were treated two times with control or WDR12 (3′ UTR)-, Pes1 (3′ UTR)-, or Bop1 (3′ UTR)-specific siRNA. Expression of HA-tagged proteins was induced 2 days after the last siRNA transfection for 6 h by addition of 0.1 μg/ml doxycycline and stopped by its removal. The stability of Pes1-HA, Bop1-HA, and WDR12-HA was analyzed by Western blotting with anti-HA antibody 3F10. α-Tubulin is shown as a loading control.
FIG. 8.
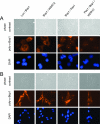
Pes1 is required for nucleolar localization of Bop1. Bop1-HA expressed in (A) H1299 cells and (B) TGR-1 cells was detected by indirect immunofluorescence assay. Coexpression of luciferase (control), WDR12, and Pes1 is indicated. Recombinant proteins were induced by doxycycline for 30 h, and cells were fixed with methanol-acetone. Bop1 was stained by guinea pig polyclonal antibodies specific for mouse Bop1 (poly-α-Bop1). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI).
FIG. 9.
Association of recombinant PeBoW components with large preribosomal particles after overexpression. Shown is a Western blot analysis of sucrose gradient fractions of TGR-1 cells expressing Bop1-HA, Pes1-HA, and WDR12-HA or coexpressing Bop1-HA/WDR12-HA and Bop1-HA/Pes1-HA. Fractions containing the 60S/pre-66S subunits (28S rRNA) are indicated.
FIG. 10.
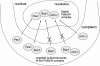
Model for transport and assembly of Pes1, Bop1, and WDR12 in the nucleolus. Cytoplasmic Pes1, Bop1, and WDR12 are unstable. Bop1 requires Pes1 for nucleolar transport, while nucleolar transport of WDR12 is blocked by high Bop1 levels. PeBoW complex formation occurs in the nucleolus and stabilizes all three proteins.
Similar articles
- The Crystal Structure of the Ubiquitin-like Domain of Ribosome Assembly Factor Ytm1 and Characterization of Its Interaction with the AAA-ATPase Midasin.
Romes EM, Sobhany M, Stanley RE. Romes EM, et al. J Biol Chem. 2016 Jan 8;291(2):882-93. doi: 10.1074/jbc.M115.693259. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601951 Free PMC article. - Dominant-negative Pes1 mutants inhibit ribosomal RNA processing and cell proliferation via incorporation into the PeBoW-complex.
Grimm T, Hölzel M, Rohrmoser M, Harasim T, Malamoussi A, Gruber-Eber A, Kremmer E, Eick D. Grimm T, et al. Nucleic Acids Res. 2006 May 31;34(10):3030-43. doi: 10.1093/nar/gkl378. Print 2006. Nucleic Acids Res. 2006. PMID: 16738141 Free PMC article. - Mammalian WDR12 is a novel member of the Pes1-Bop1 complex and is required for ribosome biogenesis and cell proliferation.
Hölzel M, Rohrmoser M, Schlee M, Grimm T, Harasim T, Malamoussi A, Gruber-Eber A, Kremmer E, Hiddemann W, Bornkamm GW, Eick D. Hölzel M, et al. J Cell Biol. 2005 Aug 1;170(3):367-78. doi: 10.1083/jcb.200501141. Epub 2005 Jul 25. J Cell Biol. 2005. PMID: 16043514 Free PMC article. - The functional role of Pescadillo ribosomal biogenesis factor 1 in cancer.
Li YZ, Zhang C, Pei JP, Zhang WC, Zhang CD, Dai DQ. Li YZ, et al. J Cancer. 2022 Jan 1;13(1):268-277. doi: 10.7150/jca.58982. eCollection 2022. J Cancer. 2022. PMID: 34976188 Free PMC article. Review. - Pre-ribosomes on the road from the nucleolus to the cytoplasm.
Tschochner H, Hurt E. Tschochner H, et al. Trends Cell Biol. 2003 May;13(5):255-63. doi: 10.1016/s0962-8924(03)00054-0. Trends Cell Biol. 2003. PMID: 12742169 Review.
Cited by
- Inactivation of Exosc10 in the oocyte impairs oocyte development and maturation, leading to a depletion of the ovarian reserve in mice.
Demini L, Kervarrec C, Guillot L, Com E, Lavigne R, Kernanec PY, Primig M, Pineau C, Petit FG, Jamin SP. Demini L, et al. Int J Biol Sci. 2023 Jan 31;19(4):1080-1093. doi: 10.7150/ijbs.72889. eCollection 2023. Int J Biol Sci. 2023. PMID: 36923944 Free PMC article. - Ribosome biogenesis factors-from names to functions.
Dörner K, Ruggeri C, Zemp I, Kutay U. Dörner K, et al. EMBO J. 2023 Apr 3;42(7):e112699. doi: 10.15252/embj.2022112699. Epub 2023 Feb 10. EMBO J. 2023. PMID: 36762427 Free PMC article. Review. - Circular RNAs in β-cell function and type 2 diabetes-related complications: a potential diagnostic and therapeutic approach.
Ghasemi H, Sabati Z, Ghaedi H, Salehi Z, Alipoor B. Ghasemi H, et al. Mol Biol Rep. 2019 Oct;46(5):5631-5643. doi: 10.1007/s11033-019-04937-x. Epub 2019 Jul 13. Mol Biol Rep. 2019. PMID: 31302804 Review. - All these screens that we've done: how functional genetic screens have informed our understanding of ribosome biogenesis.
Harold CM. Harold CM. Biosci Rep. 2023 Jul 26;43(7):BSR20230631. doi: 10.1042/BSR20230631. Biosci Rep. 2023. PMID: 37335083 Free PMC article. Review. - The Crystal Structure of the Ubiquitin-like Domain of Ribosome Assembly Factor Ytm1 and Characterization of Its Interaction with the AAA-ATPase Midasin.
Romes EM, Sobhany M, Stanley RE. Romes EM, et al. J Biol Chem. 2016 Jan 8;291(2):882-93. doi: 10.1074/jbc.M115.693259. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601951 Free PMC article.
References
- Bornkamm, G. W., C. Berens, C. Kuklik-Roos, J. M. Bechet, G. Laux, J. Bachl, M. Korndoerfer, M. Schlee, M. Hölzel, A. Malamoussi, R. D. Chapman, F. Nimmerjahn, J. Mautner, W. Hillen, H. Bujard, and J. Feuillard. 2005. Stringent doxycycline-dependent control of gene activities using an episomal one-vector system. Nucleic Acids Res. 33:e137. - PMC - PubMed
- Dez, C., and D. Tollervey. 2004. Ribosome synthesis meets the cell cycle. Curr. Opin. Microbiol. 7:631-637. - PubMed
- Dosil, M., and X. R. Bustelo. 2004. Functional characterization of Pwp2, a WD family protein essential for the assembly of the 90S pre-ribosomal particle. J. Biol. Chem. 279:37385-37397. - PubMed
- Dragon, F., J. E. Gallagher, P. A. Compagnone-Post, B. M. Mitchell, K. A. Porwancher, K. A. Wehner, S. Wormsley, R. E. Settlage, J. Shabanowitz, Y. Osheim, A. L. Beyer, D. F. Hunt, and S. J. Baserga. 2002. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417:967-970. - PubMed
- Eichler, D. C., and N. Craig. 1994. Processing of eukaryotic ribosomal RNA. Prog. Nucleic Acid Res. Mol. Biol. 49:197-239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous