Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts - PubMed (original) (raw)
Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts
Sophie Dupre-Crochet et al. Mol Cell Biol. 2007 May.
Abstract
Cadherins are the most crucial membrane proteins for the formation of tight and compact cell-cell contacts. Cadherin-based cell-cell adhesions are dynamically established and/or disrupted during various physiological and pathological processes. However, the molecular mechanisms that regulate cell-cell contacts are not fully understood. In this paper, we report a novel functional role of casein kinase 1 (CK1) in the regulation of cell-cell contacts. Firstly, we observed that IC261, a specific inhibitor of CK1, stabilizes cadherin-based cell-cell contacts, whereas the overexpression of CK1 disrupts them. CK1 colocalizes with E-cadherin and phosphorylates the cytoplasmic domain of E-cadherin in vitro and in a cell culture system. We show that the major CK1 phosphorylation site of E-cadherin is serine 846, a highly conserved residue between classical cadherins. Constitutively phosphorylated E-cadherin (S846D) is unable to localize at cell-cell contacts and has decreased adhesive activity. Furthermore, phosphorylated E-cadherin (S846D) has weaker interactions with beta-catenin and is internalized more efficiently than wild-type E-cadherin. These data indicate that CK1 is a novel negative regulator of cadherin-based cell-cell contacts.
Figures
FIG. 1.
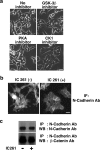
CK1 inhibitor IC261 stabilizes N-cadherin-based cell-cell contacts in HEK293 cells. (a) Effect of various kinase inhibitors on HEK293 cells. HEK293 cells were incubated with 1 μM GSK-3β inhibitor II, 200 nM H-89 (PKA inhibitor), or 10 μM IC261 (CK1 inhibitor) for 4 h. The effect was examined by phase-contrast microscopy. Scale bar, 20 μm. (b) Effect of IC261 on the localization of N-cadherin in HEK293 cells. HEK293 cells were incubated in the presence (+) or absence (−) of 10 μM IC261 for 4 h, and the localization of N-cadherin was examined by immunofluorescence with anti-N-cadherin antibody using epifluorescence microscopy. Scale bar, 20 μm. (c) Effect of IC261 on the expression of the N-cadherin complex. HEK293 cells were incubated in the presence or absence of 10 μM IC261 for 4 h, followed by immunoprecipitation (IP) with anti-N-cadherin antibody and Western blotting (WB) with anti-N-cadherin and anti-β-catenin antibodies.
FIG. 2.
CK1 inhibitor IC261 stabilizes E-cadherin-based cell-cell contacts in MCF-7 and L fibroblast cells. (a) Effect of IC261 on MCF-7 cells under low-confluence conditions. MCF-7 cells cultured at low density were incubated in the presence or absence of 10 μM IC261 for 4 h. The effect of IC261 was examined by phase-contrast and immunofluorescence (IF) microscopy with anti-E-cadherin antibody (Ab). Scale bars, 20 μm. (b and c) Effect of IC261 on low-Ca2+-induced cell separation in MCF-7 cells. MCF-7 cells were incubated in low-Ca2+ medium in the presence or absence of 10 μM IC261 for the indicated times. The effect of the treatment was examined by phase-contrast microscopy (b) and immunofluorescence microscopy with anti-β-catenin antibody (c). Scale bars, 20 μm. (d) Requirement of E-cadherin for the effect of IC261 on cell-cell contacts. L cells or L cells expressing E-cadherin were incubated in the presence or absence of 10 μM IC261 for 4 h. The effect of IC261 was examined by phase-contrast microscopy. Scale bar, 20 μm.
FIG. 3.
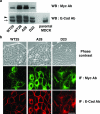
Overexpression of CK1 disrupts E-cadherin-based cell-cell contacts. The cDNA of Myc-tagged CK1α was microinjected into the nucleus of MCF-7 cells. The effect of expression of CK1α on E-cadherin-based cell-cell contacts was analyzed by immunofluorescence microscopy with the indicated antibodies. Scale bar, 40 μm.
FIG. 4.
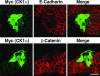
CK1 colocalizes and interacts with E-cadherin. (a and b) Colocalization between CK1α and E-cadherin in MDCK cells at steady state (a) or during Ca2+ switch (b). The subcellular localization of E-cadherin and CK1α was examined in MDCK cells using anti-E-cadherin and anti-CK1α antibodies. Scale bars, 10 μm. (c) Interaction between CK1 and E-cadherin (E-cad) by GST pull-down assays. Beads coupled to GST or the GST-tagged E-cadherin cytoplasmic domain were incubated with MCF-7 cell lysates. The proteins bound to the beads were analyzed by Coomassie protein staining and Western blotting (WB) with anti-CK1α and anti-CK1ɛ antibodies (Ab). The arrow and arrowhead indicate the positions of GST-E-cadherin and GST, respectively.
FIG. 5.
CK1 phosphorylates the cytoplasmic domain of E-cadherin. (a and b) Effect of IC261 on phosphorylation of the E-cadherin complex in MCF-7 cells in an in vivo phosphorylation assay. MCF-7 cells were cultured at low (a) or high (b) density and incubated with [32P]orthophosphate in the presence or absence of 10 μM IC261 for 2 h. In b, cells were incubated in either normal (N) or low-Ca2+ (L) medium. Cell lysates were immunoprecipitated with anti-E-cadherin antibody, followed by SDS-PAGE, autoradiography, and Western blotting (WB) with the indicated antibodies (Ab). The proteins in the autoradiography bands were identified by comparing them with the accompanying Western blotting results. (c) Phosphorylation of the cytoplasmic domain of E-cadherin by an in vitro phosphorylation assay. GST or the GST-tagged cytoplasmic domain of E-cadherin was incubated with [γ-32P]ATP in the presence of CK1δ or PKCζ. Phosphorylated proteins were subjected to SDS-PAGE, followed by autoradiography and Coomassie protein staining. (d) Amino acid sequence alignment of the cytoplasmic domains of classical cadherins. The sequence of E-, N-, OB-, VE-, and P-cadherins are from a mouse protein database. C- and DE-cadherins are E-cadherin counterparts from frog and fly, respectively. The numbers indicate the amino acid numbers for mouse E-cadherin. The potential major CK1 phosphorylation site of cadherins is in red. An analogous CK1 phosphorylation site of β-catenin is also shown. (e) Determination of the major CK1 phosphorylation site of E-cadherin by an in vitro phosphorylation assay. The GST-tagged wild type (WT) and nonphosphorylatable E-cadherin mutants were incubated with [γ-32P]ATP in the presence of CK1δ or CK2, followed by autoradiography and Coomassie protein staining. ASSS, S846A; SAAA, S849A, S852A, and S855A; AAAA, S846A, S849A, S852A, and S855A.
FIG. 6.
Mutations in the major CK1 phosphorylation residue of E-cadherin affect adhesiveness of cell-cell contacts in L fibroblast cells stably expressing E-cadherin mutants. L fibroblast clones stably expressing the Myc-tagged wild type and nonphosphorylatable (S846A) and pseudophosphorylated (S846D) mutants of E-cadherin were obtained. More than five independent clones were analyzed for each of the different types of E-cadherin, and analogous data were obtained between clones expressing the same type of E-cadherin. The data using representative clones (WT2, A5, and D13 for wild-type, S846A, and S846D E-cadherin, respectively) are shown. (a) Expression level of E-cadherin mutants in the L fibroblast clones. Cell lysates (20 μg of proteins) from the indicated clones were analyzed by Western blotting (WB) with anti-Myc antibody (Ab). (b) Effect of mutations of E-cadherin on cell-cell contact formation. Nontransfected cells or the indicated clones were cultured at high density and analyzed by phase-contrast microscopy. Scale bar, 40 μm. (c) Effect of mutations of E-cadherin on formation of cell aggregates. Nontransfected cells or the indicated clones were cultured in suspension and examined by phase-contrast microscopy. Scale bar, 40 μm. (d) Effect of mutations on subcellular localization of E-cadherin. The indicated clones were cultured at low density and analyzed by immunostaining with anti-Myc antibody. Scale bar, 20 μm. IF, immunofluorescence. (e) Effect of mutations of E-cadherin on IC261-induced stabilization of cell-cell contacts. The indicated clones were cultured at a low density in the presence or absence of 10 μM IC261 for 4 h and analyzed by phase-contrast microscopy. Scale bar, 20 μm.
FIG. 7.
Mutations in the major CK1 phosphorylation site of E-cadherin affect localization of E-cadherin in MDCK cells stably expressing E-cadherin mutants. MDCK cell clones stably expressing the Myc-tagged wild type and nonphosphorylatable (S846A) and pseudophosphorylated (S846D) mutants of E-cadherin were obtained. More than five independent clones were analyzed for each of the different types of E-cadherin, and analogous data were obtained between clones expressing the same types of E-cadherin. Data using representative clones (WT25 and WT28, A28, and D23 for wild-type, S846A, and S846D E-cadherin, respectively) are shown. (a) Expression level of E-cadherin mutants in MDCK cell clones. Cell lysates (20 μg of proteins) from the indicated clones were analyzed by Western blotting (WB) with anti-Myc and anti-E-cadherin antibodies (Ab). The arrowhead and arrow indicate the positions of exogenous Myc-tagged E-cadherin and endogenous E-cadherin, respectively. (b) Effect of mutations in the major CK1 phosphorylation site of E-cadherin on its localization in MDCK cells. The indicated clones were examined by phase-contrast microscopy and immunofluorescence (IF) microscopy with anti-Myc and anti-E-cadherin antibodies. It should be noted that anti-E-cadherin antibody detects both exogenous and endogenous E-cadherin. Scale bars, 20 μm.
FIG. 8.
CK1-catalyzed phosphorylation of E-cadherin affects the interaction between E-cadherin and β-catenin and vice versa. (a) Effect of CK1 on the interaction between E-cadherin and the binding proteins. (Upper panel) GST or the GST-tagged cytoplasmic domain of wild-type (WT) or nonphosphorylatable (S846A) E-cadherin was coupled to glutathione-Sepharose beads and incubated with ATP in the presence or absence of His6-tagged CK1δ at 30°C for 30 min. The beads were then incubated with HEK293 cell lysate at 4°C, followed by GST pull-down assays. The proteins bound to the beads were analyzed by Coomassie protein staining and Western blotting (WB) with anti-His6 antibody (Ab). (Lower panel) GST or GST-β-catenin was coupled to glutathione-Sepharose beads and incubated with His6-CK1δ, followed by GST pull-down assays. The proteins bound to the beads were analyzed by Coomassie protein staining and Western blotting with the indicated antibodies. (b) Effect of mutations of E-cadherin on its interaction with binding proteins. GST or the GST-tagged cytoplasmic domain of nonphosphorylatable (S846A) or pseudophosphorylated (S846D) E-cadherin was coupled to glutathione-Sepharose beads. The beads were then incubated with the titrated amount of HEK293 cell lysate (one-half, one-quarter, or one-eighth of cell lysate from an 80% confluent culture in a 9-cm plate). The proteins bound to the beads were analyzed by Coomassie protein staining and Western blotting with the indicated antibodies. (c) Interaction between E-cadherin and β-catenin prevents phosphorylation of E-cadherin by CK1. To uncouple β-catenin from E-cadherin, MCF-7 cells were lysed in 1% SDS lysis buffer. The lysate was then diluted 10-fold prior to immunoprecipitation (IP). Otherwise, immunoprecipitation was performed in 1% Triton X-100 lysis buffer. After immunoprecipitation, beads were intensively washed, and in vitro phosphorylation assays were performed with [γ-32P]ATP and CK1δ, followed by autoradiography and Western blotting with anti-E-cadherin, -α-catenin, and -β-catenin antibodies. Arrows indicate the positions of E-cadherin, α-catenin, and β-catenin.
FIG. 9.
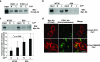
Mutations in the major CK1 phosphorylation residue of E-cadherin affect endocytosis of E-cadherin. (a) Effect of IC261 on endocytosis of endogenous E-cadherin (E-cad) in MCF-7 cells. E-cadherin in MCF-7 cells was surface biotinylated and incubated in normal (N) or low-Ca2+ (L) medium in the presence or absence of 10 μM IC261 for 5 h. Biotinylated proteins on the plasma membrane were then stripped off by glutathione treatment. Biotinylated E-cadherin inside cells was recovered on streptavidin beads and analyzed by Western blotting (WB) with anti-E-cadherin antibody (Ab). Input indicates total biotinylated E-cadherin. (b and c) Effect of E-cadherin mutation on its endocytosis in MDCK cells. (b) Wild-type and nonphosphorylatable (S846A) E-cadherin on the respective stable clones (WT28 and A28) were surface biotinylated and incubated in normal (N) or low-Ca2+ (L) medium for 8 h. After glutathione treatment, biotinylated E-cadherin inside cells was recovered on streptavidin beads and analyzed by Western blotting with anti-Myc antibody. Input indicates total biotinylated Myc-E-cadherin. (c) Wild-type E-cadherin (WT) and pseudophosphorylated E-cadherin (S846D) on the respective stable clones (WT25 and D23) were analyzed as described above, except that cells were incubated in normal (N) or low-Ca2+ (L) medium for 3 h. Input indicates total biotinylated Myc-E-cadherin. The lower panel shows the quantification of endocytosed E-cadherin compared with total biotinylated E-cadherin from the results of three independent experiments. It should be noted that different incubation times were used in b (8 h) and c (3 h) in order to maximize the difference in endocytosis between respective mutants. (d) Effect of E-cadherin mutation on its endocytosis in MDCK cells by immunofluorescence staining. Myc-tagged wild-type or pseudophosphorylated (S846D) E-cadherin was transiently expressed in MDCK cells and incubated with 2.5 μg ml−1 cycloheximide for 8 h. Cycloheximide was used to block the entry of newly synthesized E-cadherin into endosomes. The subcellular localization of exogenously expressed E-cadherin and early endosomes was analyzed using anti-Myc and anti-EEA1 antibodies. Scale bar, 20 μm. Expression of E-cadherin S846D induced flattening of cells as seen in Fig. 7b, and more early endosomes were observed in the same plane as E-cadherin in confocal microscopic analyses. Neither the E-cadherin wild type nor S846D affected the morphology of early endosomes.
Similar articles
- Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions.
Faux MC, Coates JL, Kershaw NJ, Layton MJ, Burgess AW. Faux MC, et al. PLoS One. 2010 Nov 30;5(11):e14127. doi: 10.1371/journal.pone.0014127. PLoS One. 2010. PMID: 21152425 Free PMC article. - Phosphorylation of E-cadherin at threonine 790 by protein kinase Cδ reduces β-catenin binding and suppresses the function of E-cadherin.
Chen CL, Wang SH, Chan PC, Shen MR, Chen HC. Chen CL, et al. Oncotarget. 2016 Jun 14;7(24):37260-37276. doi: 10.18632/oncotarget.9403. Oncotarget. 2016. PMID: 27203386 Free PMC article. - Functional analysis of the rodent CK1tau mutation in the circadian clock of a marine unicellular alga.
van Ooijen G, Martin SF, Barrios-Llerena ME, Hindle M, Le Bihan T, O'Neill JS, Millar AJ. van Ooijen G, et al. BMC Cell Biol. 2013 Oct 15;14:46. doi: 10.1186/1471-2121-14-46. BMC Cell Biol. 2013. PMID: 24127907 Free PMC article. - Posttranslational regulation of Neurospora circadian clock by CK1a-dependent phosphorylation.
Querfurth C, Diernfellner A, Heise F, Lauinger L, Neiss A, Tataroglu O, Brunner M, Schafmeier T. Querfurth C, et al. Cold Spring Harb Symp Quant Biol. 2007;72:177-83. doi: 10.1101/sqb.2007.72.025. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419275 Review. - Turn-off, drop-out: functional state switching of cadherins.
Lilien J, Balsamo J, Arregui C, Xu G. Lilien J, et al. Dev Dyn. 2002 May;224(1):18-29. doi: 10.1002/dvdy.10087. Dev Dyn. 2002. PMID: 11984870 Review.
Cited by
- Regulation of adherens junction dynamics by phosphorylation switches.
Bertocchi C, Vaman Rao M, Zaidel-Bar R. Bertocchi C, et al. J Signal Transduct. 2012;2012:125295. doi: 10.1155/2012/125295. Epub 2012 Jul 12. J Signal Transduct. 2012. PMID: 22848810 Free PMC article. - The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks.
Van Itallie CM, Aponte A, Tietgens AJ, Gucek M, Fredriksson K, Anderson JM. Van Itallie CM, et al. J Biol Chem. 2013 May 10;288(19):13775-88. doi: 10.1074/jbc.M113.466193. Epub 2013 Apr 3. J Biol Chem. 2013. PMID: 23553632 Free PMC article. - Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions.
Faux MC, Coates JL, Kershaw NJ, Layton MJ, Burgess AW. Faux MC, et al. PLoS One. 2010 Nov 30;5(11):e14127. doi: 10.1371/journal.pone.0014127. PLoS One. 2010. PMID: 21152425 Free PMC article. - Src and Fyn define a new signaling cascade activated by canonical and non-canonical Wnt ligands and required for gene transcription and cell invasion.
Villarroel A, Del Valle-Pérez B, Fuertes G, Curto J, Ontiveros N, Garcia de Herreros A, Duñach M. Villarroel A, et al. Cell Mol Life Sci. 2020 Mar;77(5):919-935. doi: 10.1007/s00018-019-03221-2. Epub 2019 Jul 16. Cell Mol Life Sci. 2020. PMID: 31312879 Free PMC article. - Casein kinase 1α: biological mechanisms and theranostic potential.
Jiang S, Zhang M, Sun J, Yang X. Jiang S, et al. Cell Commun Signal. 2018 May 24;16(1):23. doi: 10.1186/s12964-018-0236-z. Cell Commun Signal. 2018. PMID: 29793495 Free PMC article. Review.
References
- Bauer, A., H. Lickert, R. Kemler, and J. Stappert. 1998. Modification of the E-cadherin-catenin complex in mitotic Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 273:28314-28321. - PubMed
- Behrens, J., L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. M. Mareel, and W. Birchmeier. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120:757-766. - PMC - PubMed
- Braga, V. 2002. Cell-cell interactions: a practical approach. Oxford University Press, Oxford, England.
- Brockman, J. L., and R. A. Anderson. 1991. Casein kinase I is regulated by phosphatidylinositol 4,5-bisphosphate in native membranes. J. Biol. Chem. 266:2508-2512. - PubMed
- Carmel, G., B. Leichus, X. Cheng, S. D. Patterson, U. Mirza, B. T. Chait, and J. Kuret. 1994. Expression, purification, crystallization, and preliminary X-ray analysis of casein kinase-1 from Schizosaccharomyces pombe. J. Biol. Chem. 269:7304-7309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials