Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption - PubMed (original) (raw)
Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption
Wei Zou et al. J Cell Biol. 2007.
Abstract
In this study, we establish that the tyrosine kinase Syk is essential for osteoclast function in vitro and in vivo. Syk(-/-) osteoclasts fail to organize their cytoskeleton, and, as such, their bone-resorptive capacity is arrested. This defect results in increased skeletal mass in Syk(-/-) embryos and dampened basal and stimulated bone resorption in chimeric mice whose osteoclasts lack the kinase. The skeletal impact of Syk deficiency reflects diminished activity of the mature osteoclast and not impaired differentiation. Syk regulates bone resorption by its inclusion with the alpha v beta3 integrin and c-Src in a signaling complex, which is generated only when alpha v beta3 is activated. Upon integrin occupancy, c-Src phosphorylates Syk. Alpha v beta3-induced phosphorylation of Syk and the latter's capacity to associate with c-Src is mediated by the immunoreceptor tyrosine-based activation motif (ITAM) proteins Dap12 and FcRgamma. Thus, in conjunction with ITAM-bearing proteins, Syk, c-Src, and alpha v beta3 represent an essential signaling complex in the bone-resorbing osteoclast, and, therefore, each is a candidate therapeutic target.
Figures
Figure 1.
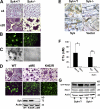
Syk deficiency impairs osteoclast function but not differentiation in vitro. (A) BMMs derived from Syk+/? or Syk−/− chimeras were cultured with RANKL and MCSF for 5 d. Cells were stained for TRAP activity (red reaction product). (B) BMMs derived from Syk+/? or Syk−/− chimeras were cultured with RANKL and MCSF on dentin for 6 d. Actin ring formation was determined by immunofluorescence after FITC-phalloidin staining. (C) After 6 d, Syk+/? or Syk−/− osteoclasts were removed, and the dentin was stained with Coomassie brilliant blue to visualize resorption lacunae. (D) Mature Syk−/− osteoclasts transduced with either WT Syk, kinase-inactive SykK402R, or pMX vector were generated on dentin for 5 d with RANKL and MCSF. Cells were stained for TRAP activity and/or with FITC-phalloidin. The expression of Syk was determined by immunoblot assay of TCL Syk−/−. (E) BMMs were retrovirally transduced with WT Syk or empty vector. Syk+/? and Syk−/− cells serve as positive and negative controls, respectively. The cells were placed on bone slices and differentiated into osteoclasts by exposure to RANKL and MCSF. After 5 d, the osteoclasts were removed. Resorption pits were visualized by incubation of the specimen with 20 μg/ml peroxidase-conjugated wheat germ agglutinin. (F) Medium was collected and assayed for CTx concentration. Actin serves as a loading control. Error bars represent SD. *, P < 0.01; **, P < 0.001. (G) Syk+/? or Syk−/− BMMs were cultured with MCSF and RANKL for 3 d. The cells were then lifted and either maintained in suspension (S) or plated on vitronectin (A) for 30 min. GTP-bound Rac1 and total Rac1 expression were determined, and the immunoblot was densitometrically quantitated.
Figure 2.
Syk deficiency results in abnormal osteoclast function in vivo. (A) PTH(1–34) or vehicle was administered to Syk+/? or Syk−/− chimeras for 4 d. Calvariae were fixed and stained for TRAP activity, and osteoclast (OC) number was histomorphometrically determined, *, P < 0.05; **, P < 0.01; PTH versus buffer. (B) TRAP-stained histological sections of calvaria of PTH-treated Syk+/? or Syk−/− chimeric mice. Osteoclasts (red reaction product) of PTH-treated Syk+/? mice reside in resorption lacunae and contain ruffled membranes (arrow), whereas most Syk−/− osteoclasts are smaller and are not juxtaposed to the bone. (C) Percentage of bone surface juxtaposed to osteoclasts in control and PTH-treated Syk+/? or Syk−/− mice. *, P < 0.05 versus Syk−/−; +, P < 0.05 versus buffer. (D) Serum was collected from vehicle and PTH-treated Syk+/? and Syk−/− chimeras. CTx was measured by ELISA. *, P < 0.01. Error bars represent SD.
Figure 3.
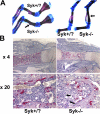
Bone density is increased in Syk−/− embryos. (A) Skeletons of 18.5-d Syk+/? and Syk−/− embryos were fixed in 95% ethanol and stained with Alcian blue and Alizarin red. Syk−/− forelimb and hindlimb bones are denser as indicated by increased Alizarin staining (arrows). (B) TRAP-stained (red reaction product) histological sections of day 18.5 Syk+/? and Syk−/− embryo femurs. Arrows indicate the trabecular network in Syk−/− but not Syk+/? marrow space.
Figure 4.
Syk does not regulate osteoblast function. (A and B) Syk+/? or Syk−/− primary calvaria osteoblasts were cultured with differentiation media (α-MEM containing 50 μg/ml ascorbic acid and 2 mM β-glycerophosphate) for 20 d. Bone nodule formation was visualized by Alizarin red staining. (C) Syk+/? or Syk−/− primary calvarial osteoblasts were cultured in α-MEM media (con), 10 nM 1,25-dihydroxyvitamin D3 (VitD), or 10 ng/ml TNF-α for 24 h. RANKL and OPG expression were analyzed by RT-PCR. Actin serves as loading control. (D) Syk+/? or Syk−/− primary calvarial osteoblasts were lysed, and Syk expression was determined by immunoblotting. WT macrophage (Mφ) lysate serves as a positive control.
Figure 5.
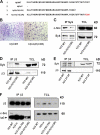
Syk, c-Src, and αvβ3 form a complex in osteoclasts. (A) β3−/− BMMs retrovirally transduced with hβ3WT were cultured with RANKL and MCSF for 5 d. Total cell lysate (TCL) was immunoprecipitated with anti-Syk or ant–c-Src antibodies or irrelevant IgG. Immunoprecipitates and TCLs were probed by Western blotting for β3-integrin, Syk, and c-Src content. (B and C) WT BMMs were cultured with MCSF and RANKL for 3 d. The cells were then lifted and either maintained in suspension (S) or plated on vitronectin (A) for 30 min. β3-integrin immunoprecipitates from each group of cells were probed by immunoblotting for β3-integrin and Syk content (B) or for c-Src content (C). (D) WT BMMs were cultured with MCSF alone or MCSF and RANKL for 3 or 5 d. TCL was immunoprecipitated with anti-Syk antibody followed by c-Src and Syk immunoblotting. (E) TCL from WT or β3−/− osteoclasts was immunoprecipitated with anti-Syk (top) or anti–c-Src (bottom) antibodies. Immunoprecipitates and TCLs were immunoblotted for c-Src and Syk content. (F) β3−/− BMMs were retrovirally transduced with hβ3 or pMX vector. The transductants were cultured in RANKL and MCSF for 5 d. Syk immunoprecipitates and TCLs were then immunoblotted for c-Src, Syk, or β3-integrin content. (G) WT preosteoclasts, which were generated by culturing BMMs in MCSF and RANKL for 3 d, were lifted and either plated on vitronectin (A) or maintained in suspension (S) for 30 min. c-Src and Syk content in Syk immunoprecipitates was determined by immunoblotting. (H) Suspended Syk+/? or Syk−/− preosteoclasts were plated on vitronectin. The percentage of spread cells was determined after 5 and 10 min. *, P < 0.001 versus Syk+/?. Error bars represent SD.
Figure 6.
The distal three amino acids of the β3-integrin cytoplasmic domain mediate Syk–c-Src association. (A) Amino acid sequences of human (h) β3-integrin constructs used in this study. Letters in red refer to substituted β1-integrin subunit residues. (B–D) β3−/− BMMs transduced with hβ3WT or hβ3–hβ1(C3R) were cultured in the presence of RANKL and MCSF for 5 d. (B) Cells were stained for TRAP activity. (C) Syk immunoprecipitates and TCLs were immunoblotted for c-Src and Syk content. (D) β3-integrin immunoprecipitates and TCLs were immunoblotted for Syk and β3-integrin content. (E) β3−/− BMMs transduced with hβ3WT or hβ3Y747F/Y752F (hβ3 2Y-2F) were cultured in the presence of RANKL and MCSF for 5 d. β3-integrin immunoprecipitates and TCLs were immunoblotted for Syk and β3-integrin content. (F) β3−/− BMMs transduced with hβ3WT, hβ3ΔC, hβ3Δ752–762, or hβ3–hβ1(C3R) were cultured in the presence of RANKL and MCSF for 5 d. β3-integrin immunoprecipitates and TCLs were immunoblotted for β3-integrin and c-Src content.
Figure 7.
c-Src mediates αvβ3-induced Syk activation in osteoclasts. (A and B) WT BMMs were cultured in RANKL and MCSF for 3 d. The preosteoclasts were lifted and preincubated in 10 μM piceatannol (Pice) or DMSO for 20 min. They were then plated on vitronectin (A) or maintained in suspension (S) for 30 min, after which tyrosine-phosphorylated Syk (A) or Vav3 (B) was determined by immunoblotting. (C) Syk−/− BMMs transduced with WT Syk, SykR195A, SykK402R, or pMX vector were cultured in RANKL and MCSF for 3 d. The preosteoclasts were lifted and plated on vitronectin for 30 min, after which phosphorylated tyrosine in Syk immunoprecipitates and TCLs was determined by immunoblotting. (D) WT BMMs were cultured in RANKL and MCSF for 3 d. The preosteoclasts were then suspended and preincubated in 2 μM SU6656 (+) or DMSO (−) for 20 min. After 30-min adhesion to vitronectin, Syk immunoprecipitates and TCLs were immunoblotted for c-Src and Syk. (E) WT BMMs cultured in RANKL and MCSF for 3 d were lifted and preincubated for 20 min in 10 μM PP2, 2 μM SU6656, or DMSO for 20 min. The cells were then plated on vitronectin (A) or maintained in suspension (S) for 30 min, after which phosphorylated tyrosine in Syk immunoprecipitates was determined by immunoblotting. (F) WT or c-Src−/− spleen cells were cultured in RANKL and MCSF for 3 d. The cells were lifted and either replated on vitronectin or maintained in suspension for 30 min, after which phosphorylated tyrosine in Syk immunoprecipitates was determined by immunoblotting. Syk, c-Src, and β-actin (loading control) content was also assessed in TCLs. (G) WT or Syk−/− BMMs were cultured in RANKL and MCSF for 3 d. The cells were lifted and either replated on vitronectin or maintained in suspension for 30 min. The plated cells were then rinsed with PBS to remove nonadherent cells. c-SrcY416 and β-actin (loading control) content was assessed by immunoblotting in TCLs of both suspended and adherent cells.
Figure 8.
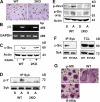
ITAM proteins mediate αvβ3-induced Syk activation in osteoclasts. (A–C) WT or Dap12−/−/FcRγ−/− (2KO) spleen cells were cultured in RANKL and MCSF for 3 d, after which the cells were stained for TRAP activity (A), analyzed for β3-integrin mRNA content by RT-PCR (B), and analyzed for c-Src expression by immunoblotting (C). (D and E) WT or Dap12−/−/FcRγ−/− (2KO) spleen cells were cultured in RANKL and MCSF for 3 d. The cells were lifted and either replated on vitronectin (A) or maintained in suspension (S) for 30 min, after which Syk immunoblots were probed for phosphotyrosine content (D) or TCLs were immunoblotted for phosphorylated Vav3 and c-Srcp-Y416 (E). (F) Syk−/− BMMs transduced with WT Syk or SykR195A were cultured in RANKL and MCSF for 5 d. Syk immunoprecipitates and TCLs were then immunoblotted for c-Src and Syk content. (G) Syk−/− BMMs were transduced with WT Syk, SykR195A, or empty vector (pMX). After 5-d exposure to RANKL and MCSF, the cells were stained for TRAP activity.
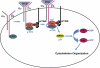
Figure 9.
Model of Syk-mediated organization of the osteoclast cytoskeleton. c-Src constitutively associates with the β3-integrin subunit. αvβ3 is activated upon ligand (RGD) occupancy, which recruits Syk bound to the Y-phosphorylated ITAM proteins FcRγ and Dap12 in an SH2 domain–dependent manner to the integrin–c-Src complex. αvβ3 occupancy also activates c-Src by phosphorylating Y416. In turn, activated c-Src phosphorylates Syk. Activated Syk phosphorylates Vav3, which shuttles Rac to its GTP-bound form, thereby organizing the actin cytoskeleton.
Similar articles
- The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase.
Mócsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. Mócsai A, et al. Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):6158-63. doi: 10.1073/pnas.0401602101. Epub 2004 Apr 8. Proc Natl Acad Sci U S A. 2004. PMID: 15073337 Free PMC article. - An osteoclastic protein-tyrosine phosphatase regulates the β3-integrin, syk, and shp1 signaling through respective src-dependent phosphorylation in osteoclasts.
Lau KH, Stiffel V, Amoui M. Lau KH, et al. Am J Physiol Cell Physiol. 2012 Jun 1;302(11):C1676-86. doi: 10.1152/ajpcell.00042.2012. Epub 2012 Mar 28. Am J Physiol Cell Physiol. 2012. PMID: 22460711 - Absence of Dap12 and the αvβ3 integrin causes severe osteopetrosis.
Zou W, Teitelbaum SL. Zou W, et al. J Cell Biol. 2015 Jan 5;208(1):125-36. doi: 10.1083/jcb.201410123. Epub 2014 Dec 29. J Cell Biol. 2015. PMID: 25547154 Free PMC article. - Role of ITAM-containing adapter proteins and their receptors in the immune system and bone.
Humphrey MB, Lanier LL, Nakamura MC. Humphrey MB, et al. Immunol Rev. 2005 Dec;208:50-65. doi: 10.1111/j.0105-2896.2005.00325.x. Immunol Rev. 2005. PMID: 16313340 Review. - Integrin-mediated signaling in the regulation of osteoclast adhesion and activation.
Duong LT, Rodan GA. Duong LT, et al. Front Biosci. 1998 Aug 1;3:d757-68. doi: 10.2741/A319. Front Biosci. 1998. PMID: 9682033 Review.
Cited by
- Interaction of key pathways in sorafenib-treated hepatocellular carcinoma based on a PCR-array.
Liu Y, Wang P, Li S, Yin L, Shen H, Liu R. Liu Y, et al. Int J Clin Exp Pathol. 2015 Mar 1;8(3):3027-35. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045814 Free PMC article. - Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions.
Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Albiges-Rizo C, et al. J Cell Sci. 2009 Sep 1;122(Pt 17):3037-49. doi: 10.1242/jcs.052704. J Cell Sci. 2009. PMID: 19692590 Free PMC article. Review. - Adaptor protein is essential for insect cytokine signaling in hemocytes.
Oda Y, Matsumoto H, Kurakake M, Ochiai M, Ohnishi A, Hayakawa Y. Oda Y, et al. Proc Natl Acad Sci U S A. 2010 Sep 7;107(36):15862-7. doi: 10.1073/pnas.1003785107. Epub 2010 Aug 23. Proc Natl Acad Sci U S A. 2010. PMID: 20798052 Free PMC article. - Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling.
Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min KH, Ivashkiv LB. Kalliolias GD, et al. Arthritis Rheum. 2010 Feb;62(2):402-13. doi: 10.1002/art.27200. Arthritis Rheum. 2010. PMID: 20112358 Free PMC article. - Spleen tyrosine kinase inhibition in the treatment of autoimmune, allergic and autoinflammatory diseases.
Pamuk ON, Tsokos GC. Pamuk ON, et al. Arthritis Res Ther. 2010;12(6):222. doi: 10.1186/ar3198. Epub 2010 Dec 17. Arthritis Res Ther. 2010. PMID: 21211067 Free PMC article. Review.
References
- Arias-Salgado, E.G., S. Lizano, S.J. Shattil, and M.H. Ginsberg. 2005. Specification of the direction of adhesive signaling by the integrin β cytoplasmic domain. J. Biol. Chem. 280:29699–29707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-078784/HL/NHLBI NIH HHS/United States
- AR032788/AR/NIAMS NIH HHS/United States
- R01 AR046523/AR/NIAMS NIH HHS/United States
- R37 AR046523/AR/NIAMS NIH HHS/United States
- AR046852/AR/NIAMS NIH HHS/United States
- AR046523/AR/NIAMS NIH HHS/United States
- AR048853/AR/NIAMS NIH HHS/United States
- P30 DK056341-06/DK/NIDDK NIH HHS/United States
- HL57900/HL/NHLBI NIH HHS/United States
- R01 AR048853/AR/NIAMS NIH HHS/United States
- MC_U117527252/MRC_/Medical Research Council/United Kingdom
- DK056341/DK/NIDDK NIH HHS/United States
- P30 DK056341-07/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 AR046852/AR/NIAMS NIH HHS/United States
- R01 AR032788/AR/NIAMS NIH HHS/United States
- R01 AR048812/AR/NIAMS NIH HHS/United States
- P01 HL078784/HL/NHLBI NIH HHS/United States
- HL78784/HL/NHLBI NIH HHS/United States
- AR048812/AR/NIAMS NIH HHS/United States
- P01 HL057900/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous