Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon - PubMed (original) (raw)
Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon
Yimin Hua et al. PLoS Biol. 2007 Apr.
Abstract
Several strategies have been pursued to increase the extent of exon 7 inclusion during splicing of SMN2 (survival of motor neuron 2) transcripts, for eventual therapeutic use in spinal muscular atrophy (SMA), a genetic neuromuscular disease. Antisense oligonucleotides (ASOs) that target an exon or its flanking splice sites usually promote exon skipping. Here we systematically tested a large number of ASOs with a 2'-O-methoxy-ethyl ribose (MOE) backbone that hybridize to different positions of SMN2 exon 7, and identified several that promote greater exon inclusion, others that promote exon skipping, and still others with complex effects on the accumulation of the two alternatively spliced products. This approach provides positional information about presumptive exonic elements or secondary structures with positive or negative effects on exon inclusion. The ASOs are effective not only in cell-free splicing assays, but also when transfected into cultured cells, where they affect splicing of endogenous SMN transcripts. The ASOs that promote exon 7 inclusion increase full-length SMN protein levels, demonstrating that they do not interfere with mRNA export or translation, despite hybridizing to an exon. Some of the ASOs we identified are sufficiently active to proceed with experiments in SMA mouse models.
Conflict of interest statement
Competing interests. TAV, BFB, and CFB are employees of Isis Pharmaceutical Corp., the owner of the antisense oligonucleotide chemistry used in this report, and materially benefit either directly or indirectly through stock options. YH and ARK, along with their employer, Cold Spring Harbor Laboratory, could materially benefit if a therapeutic for SMA results from this work. ARK serves on the scientific advisory board of two non-profit SMA foundations.
Figures
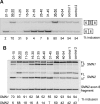
Figure 1. Initial ASO Walk along Exon 7, Assayed by In Vitro Splicing
(A) Schematic representation of the binding sites for the nine MOE ASOs used in the initial exon 7 walk. The position of complementarity of each ASO along exon 7 is indicated by a horizontal line. (B) Effect of ASOs on in vitro splicing of SMN2 minigene-derived pre-mRNA. Each ASO was tested at the indicated concentrations, and SMN1 pre-mRNA was used as a positive control. The radiolabeled RNAs were analyzed by denaturing PAGE and autoradiography. The diagrams on the right indicate the mobilities of the various RNA species. The percentage of exon 7 inclusion in each lane was calculated as described in Materials and Methods, and is indicated below each autoradiogram. The two ASOs that promote exon 7 inclusion are underlined.
Figure 2. In Vivo Validation of the Initial ASO Walk
Each ASO at a concentration of 10 μM and 2.5-μg pBabe Puro with or without 5-μg pCI-SMN2 were transfected by electroporation, and transfected cells selected with 2-μg/ml puromycin for 20 h. Two days after transfection, cells were collected for total RNA preparation, and RT-PCR was performed to analyze SMN2 pre-mRNA splicing patterns. The PCR products were labeled by incorporation of α-32P-dCTP. The ASOs that promote exon inclusion are underlined. Control 1: unrelated oligonucleotide 00–00; control 2: buffer. (A) The nine ASOs were co-transfected with pCI-SMN2, and the PCR products analyzed by 8% native PAGE. (B) The effects of the nine ASOs were analyzed with transcripts from the endogenous SMN2 gene in HEK293 cells. RT-PCR products were digested with DdeI to distinguish SMN1 from SMN2 by 6% native PAGE. The percentage of exon 7 inclusion in each lane is indicated below each autoradiogram. FL, full-length mRNA; Δ7, exon 7–deleted mRNA.
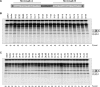
Figure 3. Two ASO Microwalks Assayed by In Vitro Splicing
(A) Nucleotides in white show the two microwalk regions. (B) Seventeen new ASOs were screened in microwalk A. The original ASO (06–20) and the improved ASO (07–21) are underlined. (C) Twenty-two new ASOs were screened in microwalk B. The original ASO (36–50) and the improved ASO (34–48) are underlined. SMN2 minigene pre-mRNA was spliced in vitro as in Figure 1, in the presence of each ASO at a concentration of 100 nM. The percentage of exon 7 inclusion in each lane is indicated below each autoradiogram.
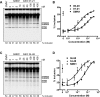
Figure 4. Dose-Response Analysis with the Two Improved ASOs Using In Vitro Splicing
ASOs 07–21 (A) and 34–48 (C) were tested at the indicated concentrations. In vitro splicing was carried out as in Figure 1. The percentage of exon 7 inclusion in each lane is indicated below each autoradiogram. (B) and (D) Sigmoidal curves were plotted to compare the dose-response effects of the high resolution–walk ASOs (A) and (C) with the initial-walk ASOs (from Figure 1) using the data from three independent experiments. The error bars show standard deviations.
Figure 5. In Vivo Validation of the Two ASO Microwalks
The effects of the 17 microwalk A ASOs were examined with the SMN2 minigene expressed in HEK293 cells (A) or with the endogenous SMN2 gene in HEK293 cells (B). The effects of the 22 microwalk B ASOs were examined with the SMN2 minigene expressed in HEK293 cells (C) or with the endogenous SMN2 gene in HEK293 cells (D). The in vivo splicing assays were carried out as in Figure 2. Control 1: unrelated oligonucleotide 00–00; control 2: buffer. The percentage of exon 7 inclusion in each lane is indicated below each autoradiogram.
Figure 6. Dose-Response and Time-Dependence Analysis with the Two Improved ASOs for Splicing of Endogenous SMN1/2 Pre-mRNAs
ASOs 07–21 (A) and 34–48 (B) were transfected into HEK293 cells, using starting concentrations from 0 to 20 μM for electroporation. Dose-response curves for ASOs 07–21 and 34–48 were plotted on the right using the data obtained from three independent experiments. Error bars indicate standard deviations. ASOs 07–21 (C) and 34–48 (D) at a concentration of 10 μM were transfected into cells by electroporation. Cells were harvested daily for 5 d to prepare total RNA for splicing analysis as in Figure 2. Exponential-decay curves were plotted on the right using the data obtained from three independent experiments. Error bars indicate standard deviations. The percentage of SMN1 and SMN2 exon 7 inclusion in each lane is indicated below each autoradiogram.
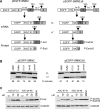
Figure 7. Effect of ASOs 07–21 and 34–48 at the mRNA and Protein Levels, Measured with Minigene Reporters
Either ASO, at a concentration of 10 or 30 μM, together with a minigene reporter plasmid, was electroporated into HEK293 cells as in Figure 2. Three days after transfection and puromycin selection, cells were harvested to generate total RNA and protein samples. (A) Diagram of the reporter constructs. pEGFP-SMN2Δ6 lacks most of exon 6, compared to pEGFP-SMN2. The alternatively spliced mRNAs are designated E7-Incl and E7-Excl, and the corresponding protein products are P-Incl and P-Excl. The natural stop codons in exons 7 and 8 are shown. (B) Total RNA samples from cells treated with 10 μM ASO were analyzed by radioactive RT-PCR with EGFP- and exon 8–specific primers. Controls include the unrelated ASO 00–00, buffer only, and an SMN1 version of the reporter minigene. The percentage of the exon-7–included isoform is indicated below each lane. (C) The proteins expressed from the reporters were detected by Western blotting with a monoclonal antibody against the HA tag; α-tubulin was detected with a monoclonal antibody as a loading control. Although exon-7–excluded mRNA expressed from the pEGFP-SMN2 plasmid was detected (B), the corresponding protein was apparently unstable (C).
Figure 8. Effect of ASOs 07–21 and 34–48 in SMA Type I Patient 3813 Fibroblasts
Endogenous SMN2 mRNA, SMN protein levels, and gem number were analyzed after each ASO at a concentration of 100 nM was transfected into patient fibroblasts using Lipofectin. Two days later, RNA or protein samples were collected, or indirect immunofluorescence microscopy was carried out. Carrier 3814 fibroblasts were used as a positive control, and ASO 00–00 and buffer treatments were used as negative controls. (A) SMN2 mRNA was analyzed by radioactive RT-PCR, using GAPDH mRNA as a loading control. The percent of SMN2 exon 7 inclusion in each lane is shown below the autoradiogram. (B) SMN protein was detected by Western blotting, and the same blot was re-probed with antibody against α-tubulin as a protein-loading control. The histograms on the right of (A) or (B) show the corresponding quantitation from three independent experiments; the ratios are normalized to that observed with the buffer control, and error bars show the standard deviations. (C) Effect of ASOs 07–21 and 34–48 on nuclear gems. Nuclei were counterstained with DAPI. Examples of treated cells with two or more gems are shown. (D and E) Both the total number of gems per 100 cells (D) and the number of cells with multiple gems (E) significantly increased after treatment with ASOs 07–21 or 34–48.
Figure 9. Schematic Diagram of the In Vivo Effects of All Tested ASOs on Exon 7 Inclusion
An essential core sequence (in bold) surrounded by two inhibitory regions, A and B (boxed), in SMN2 exon 7. Horizontal bars represent ASOs with stimulatory effects (green), inhibitory effects (red), or neutral effects (blue). The thicker the bars, the stronger the effects. Tra2β1 and another putative activator are shown bound to the central core region.
Similar articles
- Evolving concepts on human SMN pre-mRNA splicing.
Singh RN. Singh RN. RNA Biol. 2007 Jan-Mar;4(1):7-10. doi: 10.4161/rna.4.1.4535. Epub 2007 Jun 4. RNA Biol. 2007. PMID: 17592254 - Splicing of a critical exon of human Survival Motor Neuron is regulated by a unique silencer element located in the last intron.
Singh NK, Singh NN, Androphy EJ, Singh RN. Singh NK, et al. Mol Cell Biol. 2006 Feb;26(4):1333-46. doi: 10.1128/MCB.26.4.1333-1346.2006. Mol Cell Biol. 2006. PMID: 16449646 Free PMC article. - 5-(N-ethyl-N-isopropyl)-amiloride enhances SMN2 exon 7 inclusion and protein expression in spinal muscular atrophy cells.
Yuo CY, Lin HH, Chang YS, Yang WK, Chang JG. Yuo CY, et al. Ann Neurol. 2008 Jan;63(1):26-34. doi: 10.1002/ana.21241. Ann Neurol. 2008. PMID: 17924536 - The regulation and regulatory activities of alternative splicing of the SMN gene.
Singh NN, Androphy EJ, Singh RN. Singh NN, et al. Crit Rev Eukaryot Gene Expr. 2004;14(4):271-85. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.30. Crit Rev Eukaryot Gene Expr. 2004. PMID: 15663357 Review. - Spinal muscular atrophy: from gene to therapy.
Wirth B, Brichta L, Hahnen E. Wirth B, et al. Semin Pediatr Neurol. 2006 Jun;13(2):121-31. doi: 10.1016/j.spen.2006.06.008. Semin Pediatr Neurol. 2006. PMID: 17027862 Review.
Cited by
- History of development of the life-saving drug "Nusinersen" in spinal muscular atrophy.
Qiu J, Wu L, Qu R, Jiang T, Bai J, Sheng L, Feng P, Sun J. Qiu J, et al. Front Cell Neurosci. 2022 Aug 12;16:942976. doi: 10.3389/fncel.2022.942976. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36035257 Free PMC article. Review. - Therapeutic activity of modified U1 core spliceosomal particles.
Rogalska ME, Tajnik M, Licastro D, Bussani E, Camparini L, Mattioli C, Pagani F. Rogalska ME, et al. Nat Commun. 2016 Apr 4;7:11168. doi: 10.1038/ncomms11168. Nat Commun. 2016. PMID: 27041075 Free PMC article. - Mouse models of SMA: tools for disease characterization and therapeutic development.
Bebee TW, Dominguez CE, Chandler DS. Bebee TW, et al. Hum Genet. 2012 Aug;131(8):1277-93. doi: 10.1007/s00439-012-1171-5. Epub 2012 Apr 29. Hum Genet. 2012. PMID: 22543872 Review. - Antisense-mediated exon inclusion.
Hua Y, Krainer AR. Hua Y, et al. Methods Mol Biol. 2012;867:307-23. doi: 10.1007/978-1-61779-767-5_20. Methods Mol Biol. 2012. PMID: 22454070 Free PMC article. - Therapy development in spinal muscular atrophy.
Sendtner M. Sendtner M. Nat Neurosci. 2010 Jul;13(7):795-9. doi: 10.1038/nn.2565. Nat Neurosci. 2010. PMID: 20581815
References
- Munsat TL, Davies KE. International SMA consortium meeting. (26–28 June 1992, Bonn, Germany) Neuromuscul Disord. 1992;2:423–428. - PubMed
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. - PubMed
- Meister G, Buhler D, Pillai R, Lottspeich F, Fischer U. A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs. Nat Cell Biol. 2001;3:945–949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM042699/GM/NIGMS NIH HHS/United States
- R01 NS041621/NS/NINDS NIH HHS/United States
- NS041621/NS/NINDS NIH HHS/United States
- R01 GM042699/GM/NIGMS NIH HHS/United States
- GM42699/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources