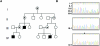Filamin A is mutated in X-linked chronic idiopathic intestinal pseudo-obstruction with central nervous system involvement - PubMed (original) (raw)
Comparative Study
doi: 10.1086/513321. Epub 2007 Feb 26.
Affiliations
- PMID: 17357080
- PMCID: PMC1852717
- DOI: 10.1086/513321
Comparative Study
Filamin A is mutated in X-linked chronic idiopathic intestinal pseudo-obstruction with central nervous system involvement
Annagiusi Gargiulo et al. Am J Hum Genet. 2007 Apr.
Abstract
We have previously reported that an X-linked recessive form of chronic idiopathic intestinal pseudo-obstruction (CIIPX) maps to Xq28. To select candidate genes for the disease, we analyzed the expression in murine fetal brain and intestine of 56 genes from the critical region. We selected and sequenced seven genes and found that one affected male from a large CIIPX-affected kindred bears a 2-bp deletion in exon 2 of the FLNA gene that is present at the heterozygous state in the carrier females of the family. The frameshift mutation is located between two close methionines at the filamin N terminus and is predicted to produce a protein truncated shortly after the first predicted methionine. Loss-of-function FLNA mutations have been associated with X-linked dominant nodular ventricular heterotopia (PVNH), a central nervous system (CNS) migration defect that presents with seizures in females and lethality in males. Notably, the affected male bearing the FLNA deletion had signs of CNS involvement and potentially has PVNH. To understand how the severe frameshift mutation we found can explain the CIIPX phenotype and its X-linked recessive inheritance, we transiently expressed both the wild- type and mutant filamin in cell culture and found that filamin translation can start from either of the two initial methionines in these conditions. Therefore, translation of a normal shorter filamin can occur in vitro from the second methionine downstream of the 2-bp insertion we found. We confirmed this, demonstrating that the filamin protein is present in the patient's lymphoblastoid cell line that shows abnormal cytoskeletal actin organization compared with normal lymphoblasts. We conclude that the filamin N terminal region between the initial two methionines is crucial for proper enteric neuron development.
Figures
Figure 1.
A, Pedigree of the CIIPX-affected family. B, Chromatogram showing FLNA exon 2 sequence around c.65-66delAC. Top, Sequence of the obligate carriers (I-1, II-1, II-5, III-2, and III-6,). Middle, Sequence of healthy male (III-1). Bottom, Sequence of the affected male (IV-1). The arrow indicates c.65-66delAC.
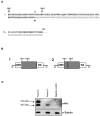
Figure 2.
Effect of c.65-66delAC on filamin A translation. A, Sequence of the filamin A NH2 terminus. Black letters indicate wild-type protein sequence; gray letters indicate predicted amino acid changes caused by c.65-66delAC; the asterisk (*) indicates a stop codon; “Met1” and “Met2” indicate the first and second filamin A methionines, respectively. B, Schematic representation of plasmids 1 and 2 expressing filamin A NH2 terminus (amino acid residues 1–124) fused to the HA under the control of the CMV promoter. The line between Met1 and Met2 in plasmid 2 indicates the position of c.65-66delAC. C, Western blot analysis of FLNA expression in HEK 293 cells transfected with plasmids 1 and 2 and in an untransfected control. Protein molecular weight is shown on the left. The primary antibodies used are shown on the right.
Figure 3.
Western blot analysis of filamin A expression in patient IV-1 lymphoblasts. The amount of total protein loaded for each individual is shown above the lanes. Protein molecular weight is shown on the left. The primary antibodies used are shown on the right.
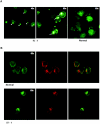
Figure 4.
Actin organization and γ-tubulin distribution in patient IV-1 lymphoblasts. A, Actin immunofluorescence with phalloidin-Texred in patient IV-1 and control lymphoblasts. The white arrows indicate the phalloidin spots, which are more abundant in patient IV-1 than in control lymphoblasts. B, Staining for actin with phalloidin-Texred (green staining) and for γ-tubulin (red staining) in patient IV-1 and control lymphoblasts. The merged image on the right shows that the phalloidin spots are on the centrosomal side of patient IV-1 lymphoblasts.
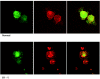
Figure 5.
Actin organization and filamin A distribution in patient IV-1 lymphoblasts. Staining for actin with phalloidin-Texred (green staining) and for filamin A (red staining) in patient IV-1 and control lymphoblasts. The merged image on the right shows that the actin organization and filamin A distribution in the cells are similar.
Similar articles
- Diffuse abnormal layering of small intestinal smooth muscle is present in patients with FLNA mutations and x-linked intestinal pseudo-obstruction.
Kapur RP, Robertson SP, Hannibal MC, Finn LS, Morgan T, van Kogelenberg M, Loren DJ. Kapur RP, et al. Am J Surg Pathol. 2010 Oct;34(10):1528-43. doi: 10.1097/PAS.0b013e3181f0ae47. Am J Surg Pathol. 2010. PMID: 20871226 - Mutation in filamin A causes periventricular heterotopia, developmental regression, and West syndrome in males.
Masruha MR, Caboclo LO, Carrete H Jr, Cendes IL, Rodrigues MG, Garzon E, Yacubian EM, Sakamoto AC, Sheen V, Harney M, Neal J, Hill RS, Bodell A, Walsh C, Vilanova LC. Masruha MR, et al. Epilepsia. 2006 Jan;47(1):211-4. doi: 10.1111/j.1528-1167.2006.00390.x. Epilepsia. 2006. PMID: 16417552 - Etiological heterogeneity of familial periventricular heterotopia and hydrocephalus.
Sheen VL, Basel-Vanagaite L, Goodman JR, Scheffer IE, Bodell A, Ganesh VS, Ravenscroft R, Hill RS, Cherry TJ, Shugart YY, Barkovich J, Straussberg R, Walsh CA. Sheen VL, et al. Brain Dev. 2004 Aug;26(5):326-34. doi: 10.1016/j.braindev.2003.09.004. Brain Dev. 2004. PMID: 15165674 - Familial cardiac valvulopathy due to filamin A mutation.
Bernstein JA, Bernstein D, Hehr U, Hudgins L. Bernstein JA, et al. Am J Med Genet A. 2011 Sep;155A(9):2236-41. doi: 10.1002/ajmg.a.34132. Epub 2011 Aug 3. Am J Med Genet A. 2011. PMID: 21815255 Review. - Filamin-a-related myxomatous mitral valve dystrophy: genetic, echocardiographic and functional aspects.
Lardeux A, Kyndt F, Lecointe S, Marec HL, Merot J, Schott JJ, Le Tourneau T, Probst V. Lardeux A, et al. J Cardiovasc Transl Res. 2011 Dec;4(6):748-56. doi: 10.1007/s12265-011-9308-9. Epub 2011 Jul 20. J Cardiovasc Transl Res. 2011. PMID: 21773876 Review.
Cited by
- Pediatric Intestinal Pseudo-obstruction in the Era of Genetic Sequencing.
Gamboa HE, Sood M. Gamboa HE, et al. Curr Gastroenterol Rep. 2019 Dec 17;21(12):70. doi: 10.1007/s11894-019-0737-y. Curr Gastroenterol Rep. 2019. PMID: 31848803 Review. - Pediatric Intestinal Pseudo-Obstruction: Progress and Challenges.
Turcotte MC, Faure C. Turcotte MC, et al. Front Pediatr. 2022 Apr 13;10:837462. doi: 10.3389/fped.2022.837462. eCollection 2022. Front Pediatr. 2022. PMID: 35498768 Free PMC article. - Enteric Neuromyopathies: Highlights on Genetic Mechanisms Underlying Chronic Intestinal Pseudo-Obstruction.
Bianco F, Lattanzio G, Lorenzini L, Mazzoni M, Clavenzani P, Calzà L, Giardino L, Sternini C, Costanzini A, Bonora E, De Giorgio R. Bianco F, et al. Biomolecules. 2022 Dec 10;12(12):1849. doi: 10.3390/biom12121849. Biomolecules. 2022. PMID: 36551277 Free PMC article. Review. - Enteric nervous system development: migration, differentiation, and disease.
Lake JI, Heuckeroth RO. Lake JI, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Jul 1;305(1):G1-24. doi: 10.1152/ajpgi.00452.2012. Epub 2013 May 2. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23639815 Free PMC article. Review. - The MECP2 duplication syndrome.
Ramocki MB, Tavyev YJ, Peters SU. Ramocki MB, et al. Am J Med Genet A. 2010 May;152A(5):1079-88. doi: 10.1002/ajmg.a.33184. Am J Med Genet A. 2010. PMID: 20425814 Free PMC article. Review.
References
Web Resources
- Human Genome Browser Gateway, http://genome.ucsc.edu/cgi-bin/hgGateway (for the CIIPX critical region)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CIIP, HSCR, FLNA, ATP2B3, DUSP9, SLC6A8, ABCD1, L1CAM, and PVNH)
References
- Milla PJ (1994) Intestinal pseudo-obstruction in children. Wrightson Biomedical, Petersfield, United Kingdom, and Bristol, PA
- Staiano A, Tozzi A, Auricchio A (1996) Aetiology and assessment of constipation in children. In: Barbara L, Corindolesi R, Gizzi G, Stanghellini V (eds) Chronic constipation. Saunders Company, Philadelphia, pp 153–168
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous