Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord - PubMed (original) (raw)
Comparative Study
Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord
Chian-Yu Peng et al. Neuron. 2007.
Abstract
The ventral spinal cord generates multiple inhibitory and excitatory interneuron subtypes from four cardinal progenitor domains (p0, p1, p2, p3). Here we show that cell-cell interactions mediated by the Notch receptor play a critical evolutionarily conserved role in the generation of excitatory v2aIN and inhibitory v2bIN interneurons. Lineage-tracing experiments show that the v2aIN and v2bIN develop from genetically identical p2 progenitors. The p2 daughter cell fate is controlled by Delta4 activation of Notch receptors together with MAML factors. Cells receiving Notch signals activate a transcription factor code that specifies the v2bIN fate, whereas cells deprived of Notch signaling express another code for v2aIN formation. Thus, our study provides insight into the cell-extrinsic signaling that controls combinatorial transcription factor profiles involved in regulating the process of interneuron subtype diversification.
Figures
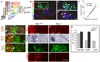
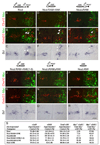
Figure 1. Generation of v2aIN and v2bIN from Lhx3+ p2 Progenitors Is Regulated by Notch Receptor Signaling
(A) Summary of v2IN development. (B) Analysis of e11.5, Lhx3:IRES:CRE; β-actin-LNL-nLacZ mice. Transverse section shows the expression of β-galactosidase (blue), Chx10, and Gata3 in the p2 domain (boxed area). The midline is to the left and ventral is to the bottom in this and all subsequent panels. Blue cells outside the p2 domain are motor neurons. (B′ and B″) Higher-magnification view of the boxed area in panel (B). Colocalization of β-Gal with Chx10 ([B′], arrowheads) and Gata3 ([B″] arrowheads) is evident in many cells. (C) Chx10 and Gata3 cell counts in wild-type mice (mean cell count ± SEM; n = 5 embryos). Fewer v2bIN are seen at e13.5 since Gata3 expression is lost during development, whereas Chx10 expression is maintained even in adult mice. (D) Expression of Lhx3 and Gata2 in e11.5 wild-type mouse spinal cord. Colocalization of Lhx3 and Gata2 (yellow nuclei, arrow) is evident in the p2-VZ but not in p2-ML. (E and F) Coexpression of Nicd and Lhx3 (E) or Gata2 (F) in some p2 progenitors (arrows) but not in others (arrowheads). The identity of the few Lhx3 and Gata2 cells that do not show detectable expression of Nicd is not clear (see Figure 8 for a possible explanation). (G and G′) Expression of Nicd is greatly reduced in _PS1_−/− mice ([G′], n = 5 embryos) when compared to controls ([G], n = 6 embryos). (H and H′) Delta4 expression is moderately increased in the _PS1_−/− mice (arrow, [H and H′]). Delta4 is also expressed in blood vessels located throughout the spinal cord (arrowhead in [H′]). (I and I′) More Chx10 neurons are generated in the _PS1_−/− embryos (I′) compared to the controls (I). (J and J′) Expression of Gata3 is seen in the p2-ML of the spinal cord from the PS1+/+ mice (J). Gata3 expression is lost in the _PS1_−/− mice (J′). (K) Cell count of total v2IN (Chx10 + Gata3 cells), v2aIN, and v2bIN in PS1+/+ and _PS1_−/− mice (mean ± SEM; ten sections per embryo; n = 6 for PS1+/+ embryos; n = 5 for _PS1_−/− embryos). The total number of v2IN is increased (121% of the control number, p = 0.0004), whereas the number of v2aIN is almost doubled (217% of the control number, p = 1.12e−31). Note that v2bIN are not generated in _PS1_−/− mice. Scale bar, 75 µm in (B), 50 µm in (B′)–(J′).
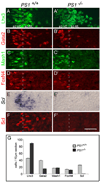
Figure 2. Reduced Notch Signaling in PS1−/− Embryos Leads to the Loss of Scl Expression in the p2 Domain
(A–F) Expression of transcription factors in the p2 domain of e11.5 PS1+/+ and _PS1_−/− mice. In PS1+/+ embryos, Lhx3 (A), Gata2 (B), Mash1 (C), FoxN4 (D), Scl mRNA (E), and Scl protein (F) expression is evident in the p2 domain. In the _PS1_−/− embryos, Lhx3 (A′), Gata2 (B′), Mash1 (C′), and FoxN4 (D′) expression is retained, whereas Scl mRNA (E′) and Scl protein are not detected (F′). Note that Gata2 expression is reduced in the p2-VZ and absent from the p2-ML in _PS1_−/− embryos (B′). (G) Cell counts (mean ± SEM; ten sections per embryo; n = 6 for PS1+/+ embryos; n = 5 for _PS1_−/− embryos) in the spinal cord of e11.5 PS1+/+ and _PS1_−/− embryos. The number of Lhx3 cells is increased (p = 8.6e−26), Gata2 cells are decreased (p = 1.6e−43), and the number of Mash1 or FoxN4 cells is not changed (p > 0.1). In the _PS1_−/− mice, Scl-expressing cells are not detected. Scale bar, 50 µm.
Figure 3. Changes in Combinatorial Gene Expression in the p2-VZ of PS1−/− Mice Reveal the Role of Notch Signaling in v2aIN versus v2bIN Cell-Fate Program
(A–D) Expression of Scl protein in the p2 domain of e11.5 wild-type mice. Scl is coexpressed with Gata2 in the p2-VZ and p2-ML ([A], arrows). Lhx3 ([B], arrow) and Gata3 ([C], arrow), but not with Chx10 (D). Arrowheads indicate cells that express only Gata2 (A) or Scl (C). (E) Cell counts of combinatorial expression of Scl/Lhx3 and Scl/Gata2 in the p2-VZ show that a small but significant number of Scl cells coexpress Lhx3 (21.56% of Scl cells, n = 388 cells analyzed in six embryos). In contrast, all Scl cells coexpress Gata2 (100% of Scl cells, n = 399 cells analyzed in six embryos). (F–H) Lhx3/Gata2 (F and F′), Gata2/Mash1 (G and G′), and Lhx3/Mash1 (H and H′) are coexpressed in the p2 domain of e11.5 PS1+/+ and _PS1_−/− embryos. The numbers (mean ± SEM; ten sections per embryo; n = 6 embryos) of Lhx3/Gata2 ([F″], p = 0.56), Gata2/Mash1 ([G″], p = 0.61), and Lhx3/Mash1 ([H″], p = 0.19) and Mash1-alone cells ([G″], p = 0.67; [H″], p = 0.49) are not affected in the _PS1_−/− embryos. However, Lhx3-alone cells are increased ([F″], p = 5.6e−5; [H″], p = 8.7e−6) and Gata2-alone cells are decreased ([F″], p = 2.5e−14; [G″], p = 8.2e−9) in the p2-VZ of _PS1_−/− embryos. (I) A schematic model that summarizes the p2 progenitor cell types identified in PS1+/+ and _PS1_−/−embryos. Scale bar, 50 µm.
Figure 4. Coexpression of Lhx3, Gata2, and Mash1, but Not Scl, in Mitotic p2 Progenitors
(A–H) Temporal sequence of transcription factor expression in the p2 domain. Following 1 hr pulse, many BrdU-positive cells in the p2 domain coexpress Gata2 (A), Lhx3 (B), and Mash1 (C), but colocalization with Scl is not observed (D). The p2 progenitors enter the mitotic phase starting 3 hr after BrdU incorporation, as revealed by coexpression of Phospho-Histone H3 ([E], ph-H3, arrow). Coexpression of Scl is observed following a 6 hr BrdU pulse ([F], arrow). The v2aIN and v2bIN are labeled with BrdU only after a 10 hr pulse ([G], arrow), suggesting that Scl expression precedes the v2bIN cell fate. None of the mpm2-marked mitotic cells in the p2 domain express Scl ([H], arrowhead). (I–K) Triple-labeling studies show coexpression of Lhx3/Gata2 (I), Lhx3/Mash1 (J), and Gata2/Mash1 (K) in mitotic cells labeled with mpm2 antibody (blue, arrow). Note that most mpm2-marked cells that express one factor also express the other. However, some mpm2-marked cells do not express either factor tested. (L) Model shows the sequence of combinatorial gene expression in the p2 progenitors. Scale bar, 50 µm.
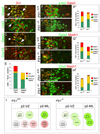
Figure 5. Activation of Notch Signaling by Delta4 Promotes the v2bIN Cell Fate at the Expense of v2aIN Cell Fate in the Chick Spinal Cord
(A–D) In stage 24 chick thoracic spinal cord, expression of Notch1, Delta1, and Delta4 is seen in Lhx3 cells within the p2-VZ ([A–C], arrowheads), but Jagged1 is expressed dorsal to the p2 domain (D). (E–J) Electroporation studies in the chick spinal cord show that Delta4, but not Delta1, promotes v2bIN cell fate. Representative transverse sections from stage 25 chick thoracic spinal cord after electroporation of Delta1 and Delta4 at stage13. Electroporated side is to the right in these and subsequent panels. Expression of the transgene is shown in green ([E and F], GFP; [H and I], β-galactosidase). Electroporation of Delta1 does not alter the Chx10 (E), Gata2/3 (F), or Scl (G) expression. Electroporation of Delta4 results in the generation of fewer Chx10 (H) and more Gata2/3 neurons ([I]; arrowheads). Delta4 also upregulates the expression of Scl on the electroporated side ([J], arrowheads). (K) Cell counts (mean ± SEM) in embryos electroporated with Notch1 (n = 5 embryos), Delta1 (n = 8 embryos), or Delta4 (n = 8 embryos). (L and M) In situ hybridization for Delta4 or Delta1 in chick embryo spinal cord following electroporation of Mash1. Ectopic Mash1 promotes the expression of Delta4 expression ([E], n = 4 embryos) but inhibits the expression of Delta1 ([F], n = 4 embryos). Scale bar, 20 µm in (A)–(D); 50 µm in (E)–(J), (L), and (M).
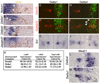
Figure 6. Deletion Analysis Reveals that the Ankyrin Repeat Seven Is Required for Nicd to Activate the v2bIN Cell-Fate Program
Representative transverse sections from stage 25 chick thoracic spinal cord after electroporation of Nicd and Nicd-deletion constructs at stage 13. Double labels for Chx10 or Gata2/3 with the electroporated transgene and the expression of Scl are shown. (A–C) In embryos electroporated with Nicd ([A–C], n = 10 embryos), Nicd:RAM+ANK ([D–F], n = 10 embryos), and NicdΔRAM ([G–I], n = 10 embryos), fewer Chx10 and more Gata2/3 and _Scl_expressing neurons are present in the electroporated side. In the electroporated side, some ectopic v2bIN appear to have migrated to a more dorsal location. Changes in v2IN cell fate are not observed in Nicd:RAM+ANK(1–6) ([J–L]; n = 8 embryos), NicdΔRAMΔANK ([M–O]; n = 7 embryos), or Nicd:ANK ([P–R]; n = 8 embryos). (S) Quantification of v2aIN and v2bIN cell counts in electroporated embryos. Changes in v2IN cell fate are presented as the ratio of the mean cell numbers on the electroporated side over the control side ± SEM, calculated from ten sections per embryo. Same embryos are analyzed for the number of myc+ v2aIN and v2bIN compared to the number of total myc+ v2IN population. Scale bar, 50 µm.
Figure 7. Nicd-MAML Complex Is Required for the Activation of the Scl-Dependent v2bIN Cell-Fate Program
(A–K) Transverse sections from stage 25 chick thoracic spinal cord following electroporation with DN-MAML1 ([A–C], n = 8 embryos), DN-MAML1+ Nicd ([D–F], n = 8 embryos), DN-MAML1+Scl ([G–I], n = 6 embryos), and Scl ([J and K], n = 10 embryos). More Chx10 neurons and fewer Gata2/3 neurons are generated in embryos electroporated with DN-MAML1 (A and B). DN-MAML1 also inhibits Scl expression (C). Coelectroporation of DN-MAML1 and Nicd also results in more Chx10 (D) and fewer Gata2/3 (E) and Scl (F) expressing cells on the electroporated side. Coelectroporation of DN-MAML1 and Scl rescues the DN-MAML1 phenotype as more v2aIN and v2bIN are generated (G and H). Scl expression is also promoted in the ectopic v2bIN generated as the result (I). Electroporation of Scl alone results in fewer v2aIN and more v2bIN (J and K). (L) Quantification of v2aIN and v2bIN cell count in electroporated embryos. Changes in v2IN cell fate are presented as the ratio of the mean cell numbers on the electroporated side over the control side ± SEM in one-half of the thoracic spinal cord, calculated from ten sections per embryo.
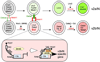
Figure 8. Schematic Model Diagram of Notch-Mediated v2aIN and v2bIN Cell-Fate Specification
This model shows the development of p2 progenitors starting with Lhx3 expression, generation of identifiable transient progenitors and leading to the generation of v2aIN or v2bIN. Salient features of this lineage include the following: (1) lower expression of Nicd in the early common progenitor as it enters terminal differentiation; (2) coexpression of four transcription factors (Lhx3, Gata2, Mash1, and FoxN4) in the mitotic, common p2 progenitor; (3) likely expression of Delta4 in the common progenitors and during the initial steps in the v2aIN lineage; (4) activation of the Notch receptor in the v2bIN lineage, leading to high Nicd in v2bIN progenitors and low Nicd in v2aIN progenitors; (5) Nicd-and MAML-dependent activation of Scl expression in the v2bIN lineage. These four steps generate nearly equal numbers of v2aIN and v2bIN from the p2 progenitors in the mouse and chick spinal cord.
Similar articles
- Asymmetric activation of Dll4-Notch signaling by Foxn4 and proneural factors activates BMP/TGFβ signaling to specify V2b interneurons in the spinal cord.
Misra K, Luo H, Li S, Matise M, Xiang M. Misra K, et al. Development. 2014 Jan;141(1):187-98. doi: 10.1242/dev.092536. Epub 2013 Nov 20. Development. 2014. PMID: 24257627 Free PMC article. - Different combinations of Notch ligands and receptors regulate V2 interneuron progenitor proliferation and V2a/V2b cell fate determination.
Okigawa S, Mizoguchi T, Okano M, Tanaka H, Isoda M, Jiang YJ, Suster M, Higashijima S, Kawakami K, Itoh M. Okigawa S, et al. Dev Biol. 2014 Jul 15;391(2):196-206. doi: 10.1016/j.ydbio.2014.04.011. Epub 2014 Apr 24. Dev Biol. 2014. PMID: 24768892 - Notch signaling regulates neural precursor allocation and binary neuronal fate decisions in zebrafish.
Shin J, Poling J, Park HC, Appel B. Shin J, et al. Development. 2007 May;134(10):1911-20. doi: 10.1242/dev.001602. Epub 2007 Apr 18. Development. 2007. PMID: 17442701 - Mastermind-like transcriptional co-activators: emerging roles in regulating cross talk among multiple signaling pathways.
McElhinny AS, Li JL, Wu L. McElhinny AS, et al. Oncogene. 2008 Sep 1;27(38):5138-47. doi: 10.1038/onc.2008.228. Oncogene. 2008. PMID: 18758483 Review. - Insights into the mechanisms of neuron generation and specification in the zebrafish ventral spinal cord.
Cucun G, Köhler M, Pfitsch S, Rastegar S. Cucun G, et al. FEBS J. 2024 Feb;291(4):646-662. doi: 10.1111/febs.16913. Epub 2023 Aug 3. FEBS J. 2024. PMID: 37498183 Review.
Cited by
- Molecular and cellular development of spinal cord locomotor circuitry.
Lu DC, Niu T, Alaynick WA. Lu DC, et al. Front Mol Neurosci. 2015 Jun 16;8:25. doi: 10.3389/fnmol.2015.00025. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26136656 Free PMC article. Review. - Lineage programming: navigating through transient regulatory states via binary decisions.
Bertrand V, Hobert O. Bertrand V, et al. Curr Opin Genet Dev. 2010 Aug;20(4):362-8. doi: 10.1016/j.gde.2010.04.010. Epub 2010 May 27. Curr Opin Genet Dev. 2010. PMID: 20537527 Free PMC article. Review. - SLUG and Truncated TAL1 Reduce Glioblastoma Stem Cell Growth Downstream of Notch1 and Define Distinct Vascular Subpopulations in Glioblastoma Multiforme.
Guelfi S, Orsetti B, Deleuze V, Rigau V, Bauchet L, Duffau H, Rothhut B, Hugnot JP. Guelfi S, et al. Cancers (Basel). 2021 Oct 27;13(21):5393. doi: 10.3390/cancers13215393. Cancers (Basel). 2021. PMID: 34771555 Free PMC article. - Asymmetric activation of Dll4-Notch signaling by Foxn4 and proneural factors activates BMP/TGFβ signaling to specify V2b interneurons in the spinal cord.
Misra K, Luo H, Li S, Matise M, Xiang M. Misra K, et al. Development. 2014 Jan;141(1):187-98. doi: 10.1242/dev.092536. Epub 2013 Nov 20. Development. 2014. PMID: 24257627 Free PMC article. - Generation of highly enriched V2a interneurons from mouse embryonic stem cells.
Iyer NR, Huettner JE, Butts JC, Brown CR, Sakiyama-Elbert SE. Iyer NR, et al. Exp Neurol. 2016 Mar;277:305-316. doi: 10.1016/j.expneurol.2016.01.011. Epub 2016 Jan 16. Exp Neurol. 2016. PMID: 26784005 Free PMC article.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
- Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. - PubMed
- Benedito R, Duarte A. Expression of Dll4 during mouse embryogenesis suggests multiple developmental roles. Gene Expr. Patterns. 2005;5:750–755. - PubMed
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous