Dopamine modulation of neuronal Na(+) channels requires binding of A kinase-anchoring protein 15 and PKA by a modified leucine zipper motif - PubMed (original) (raw)
Dopamine modulation of neuronal Na(+) channels requires binding of A kinase-anchoring protein 15 and PKA by a modified leucine zipper motif
W Preston Few et al. Proc Natl Acad Sci U S A. 2007.
Abstract
In hippocampal pyramidal cells, dopamine acts at D1 receptors to reduce peak Na(+) currents by activation of phosphorylation by PKA anchored via an A kinase-anchoring protein (AKAP15). However, the mechanism by which AKAP15 anchors PKA to neuronal Na(+) channels is not known. By using a strategy of coimmunoprecipitation from transfected tsA-201 cells, we have found that AKAP15 directly interacts with Na(v)1.2a channels via the intracellular loop between domains I and II. This loop contains key functional phosphorylation sites. Mutagenesis indicated that this interaction occurs through a modified leucine zipper motif near the N terminus of the loop. Whole-cell patch clamp recordings of acutely dissociated hippocampal pyramidal cells revealed that the D1 dopamine receptor agonist SKF 81297 reduces peak Na(+) current amplitude by 20.5%, as reported previously. Disruption of the leucine zipper interaction between Na(v)1.2a and AKAP15 through the inclusion of a small competing peptide in the patch pipette inhibited the SKF 81297-induced reduction in peak Na(+) current, whereas a control peptide with mutations in amino acids important for the leucine zipper interaction did not. Our results define the molecular mechanism by which G protein-coupled signaling pathways can rapidly and efficiently modulate neuronal excitability through local protein phosphorylation of Na(+) channels by specifically anchored PKA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
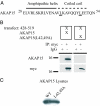
Interaction of LI–II of Nav1.2a with AKAP15. (A) Schematic diagram of myc-tagged LI–II constructs with N-terminal or C-terminal lipid anchors. Numbers refer to amino acids. (B) (Upper) Blots show coimmunoprecipitation of AKAP15 detected by using anti-AKAP15 (25) with the whole LI–II loop (_p_428–753) and _p_428–519 immunoprecipitated by using an anti-myc Ab or nonimmune IgG, as indicated. (Lower) Blots reprobed with anti-myc Ab to confirm immunoprecipitation (IP) of constructs. The faint band in the bottom left blot (55 kDa) is the heavy chain of the Ab used for immunoprecipitation.
Fig. 2.
Interaction of AKAP15 with the N terminus of LI–II of Nav1.2a via a modified leucine zipper motif. (A) The amphipathic helix and modified leucine zipper motif of AKAP15. Underlined leucines at positions 42 and 49 were mutated to alanine. (B) (Upper) Blots show coimmunoprecipitation of WT AKAP15 or AKAP15(L42,49A) with myc-tagged _p_428–519, as indicated. (Lower) Blots reprobed with anti-myc Ab to confirm adequate immunoprecipitation (IP) of _p_428–519. (C) Lysates from transfected cells probed with AKAP15 Ab demonstrating approximately equal expression of WT and mutant (L42,49A) AKAP15.
Fig. 3.
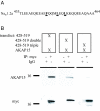
Binding of AKAP15 to the WT or mutant N terminus (amino acids 428–519) of LI–II of Nav1.2a (A) The modified leucine zipper motif of Nav1.2a. (B) (Upper) Blots show coimmunoprecipitation of AKAP15 with myc-tagged WT and mutant _p_428–519 or IgG, from transfected tsA-201 cells, as indicated. (Lower) Blots reprobed with anti-myc Ab to confirm similar immunoprecipitation (IP) of _p_428–519, the double mutant, and the triple mutant.
Fig. 4.
Modulation of hippocampal Na+ current by a dopaminergic agonist. (A) Normalized peak Na+ current is reduced by SKF 81297 application. Currents were elicited by a 40-ms pulse to −20 mV from a holding potential of −70 mV once every 2 s. (B) Example Na+ currents from the same cell as in A before and after SKF 81297 perfusion. (C) Control experiment demonstrating stability of peak current amplitude in the absence of SKF 81297 perfusion. (D) Example Na+ currents from the same cell as in C.
Fig. 5.
Block of dopaminergic modulation of hippocampal Na+ current by a peptide inhibitor of the AKAP15/Nav1.2 leucine zipper interaction. (A) Effect of intracellular dialysis of AKAP15 LZ peptide (100 μM) on SKF 81297 reduction of Na+ current amplitude. Currents were elicited by a 40-ms pulse to −20 mV from a holding potential of −70 mV once every 2 s. (B) Example Na+ currents from the same cell as in A before and after SKF 81297 perfusion. (C) Efect of intracellular dialysis of AKAP15 LZM peptide (100 μM) on SKF 81297 reduction of Na+ current amplitude. (D) Example Na+ currents from the same cell as in C before and after SKF 81297 perfusion. (E) Bar graph summarizing data from control (no peptide) and peptide experiments.
Fig. 6.
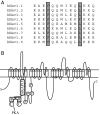
Conserved leucine zipper-like motifs in the Nav1 family of voltage-gated Na+ channels. (A) Amino acid sequence alignment of the leucine zipper-like region identified in Nav1.2 with other members of the Nav1 family. (B) Model of the leucine zipper-like interaction between AKAP15 and LI–II of Nav1.2. AKAP15 directly interacts with the N terminus of LI–II in close proximity to the PKA phosphorylation sites.
Similar articles
- Molecular mechanism of convergent regulation of brain Na(+) channels by protein kinase C and protein kinase A anchored to AKAP-15.
Cantrell AR, Tibbs VC, Yu FH, Murphy BJ, Sharp EM, Qu Y, Catterall WA, Scheuer T. Cantrell AR, et al. Mol Cell Neurosci. 2002 Sep;21(1):63-80. doi: 10.1006/mcne.2002.1162. Mol Cell Neurosci. 2002. PMID: 12359152 - Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15.
Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Hulme JT, et al. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):13093-8. doi: 10.1073/pnas.2135335100. Epub 2003 Oct 20. Proc Natl Acad Sci U S A. 2003. PMID: 14569017 Free PMC article. - Dopaminergic modulation of voltage-gated Na+ current in rat hippocampal neurons requires anchoring of cAMP-dependent protein kinase.
Cantrell AR, Tibbs VC, Westenbroek RE, Scheuer T, Catterall WA. Cantrell AR, et al. J Neurosci. 1999 Sep 1;19(17):RC21. doi: 10.1523/JNEUROSCI.19-17-j0003.1999. J Neurosci. 1999. PMID: 10460275 Free PMC article. - Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.
Catterall WA. Catterall WA. Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review. - Regulation of neuronal PKA signaling through AKAP targeting dynamics.
Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Dell'Acqua ML, et al. Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
Cited by
- Activation of histamine type 2 receptors enhances intrinsic excitability of medium spiny neurons in the nucleus accumbens.
Aceto G, Nardella L, Nanni S, Pecci V, Bertozzi A, Colussi C, D'Ascenzo M, Grassi C. Aceto G, et al. J Physiol. 2022 May;600(9):2225-2243. doi: 10.1113/JP282962. Epub 2022 Apr 13. J Physiol. 2022. PMID: 35343587 Free PMC article. - Altered excitatory and decreased inhibitory transmission in the prefrontal cortex of male mice with early developmental disruption to the ventral hippocampus.
Nath M, Bhardwaj SK, Srivastava LK, Wong TP. Nath M, et al. Cereb Cortex. 2023 Jan 5;33(3):865-880. doi: 10.1093/cercor/bhac107. Cereb Cortex. 2023. PMID: 35297476 Free PMC article. - HERV-W Envelope Triggers Abnormal Dopaminergic Neuron Process through DRD2/PP2A/AKT1/GSK3 for Schizophrenia Risk.
Yan Q, Wu X, Zhou P, Zhou Y, Li X, Liu Z, Tan H, Yao W, Xia Y, Zhu F. Yan Q, et al. Viruses. 2022 Jan 14;14(1):145. doi: 10.3390/v14010145. Viruses. 2022. PMID: 35062349 Free PMC article. - Dopaminergic Transmission Rapidly and Persistently Enhances Excitability of D1 Receptor-Expressing Striatal Projection Neurons.
Lahiri AK, Bevan MD. Lahiri AK, et al. Neuron. 2020 Apr 22;106(2):277-290.e6. doi: 10.1016/j.neuron.2020.01.028. Epub 2020 Feb 18. Neuron. 2020. PMID: 32075716 Free PMC article. - Basal Ganglia Neuromodulation Over Multiple Temporal and Structural Scales-Simulations of Direct Pathway MSNs Investigate the Fast Onset of Dopaminergic Effects and Predict the Role of Kv4.2.
Lindroos R, Dorst MC, Du K, Filipović M, Keller D, Ketzef M, Kozlov AK, Kumar A, Lindahl M, Nair AG, Pérez-Fernández J, Grillner S, Silberberg G, Hellgren Kotaleski J. Lindroos R, et al. Front Neural Circuits. 2018 Feb 6;12:3. doi: 10.3389/fncir.2018.00003. eCollection 2018. Front Neural Circuits. 2018. PMID: 29467627 Free PMC article. Review.
References
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 2001.
- Cantrell AR, Catterall WA. Nat Rev Neurosci. 2001;2:397–407. - PubMed
- Costa MR, Casnellie JE, Catterall WA. J Biol Chem. 1982;257:7918–7921. - PubMed
- Costa MR, Catterall WA. J Biol Chem. 1984;259:8210–8218. - PubMed
- Rossie S, Catterall WA. J Biol Chem. 1987;262:12735–12744. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials