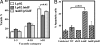Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family - PubMed (original) (raw)
Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family
Simran Banga et al. Proc Natl Acad Sci U S A. 2007.
Abstract
To establish a vacuole that supports bacterial replication, Legionella pneumophila translocates a large number of bacterial proteins into host cells via the Dot/Icm type IV secretion system. Functions of most of these translocated proteins are unknown, but recent investigations suggest their roles in modulating diverse host processes such as vesicle trafficking, autophagy, ubiquitination, and apoptosis. Cells infected by L. pneumophila exhibited resistance to apoptotic stimuli, but the bacterial protein directly involved in this process remained elusive. We show here that SidF, one substrate of the Dot/Icm transporter, is involved in the inhibition of infected cells from undergoing apoptosis to allow maximal bacterial multiplication. Permissive macrophages harboring a replicating sidF mutant are more apoptotic and more sensitive to staurosporine-induced cell death. Furthermore, cells expressing SidF are resistant to apoptosis stimuli. SidF contributes to apoptosis resistance in L. pneumophila-infected cells by specifically interacting with and neutralizing the effects of BNIP3 and Bcl-rambo, two proapoptotic members of Bcl2 protein family. Thus, inhibiting the functions of host pro-death proteins by translocated effectors constitutes a mechanism for L. pneumophila to protect host cells from apoptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Phenotypes associated with infection by the sidF mutant. (A) The sidF mutant formed fewer large phagosomes. Mouse macrophages were infected at an MOI of 1, and the distribution of vacuoles was determined at 14 h after infection as described (32). Strains tested are Lp02(intact dot/icm), Lp03(dotA_−) (data not shown), Lp02ΔsidF, and Lp02ΔsidF harboring pZL623(pSidF). Data are from two independent experiments performed in triplicate, and >150 vacuoles were scored in each sample. (B) Mouse macrophages infected with a sidF mutant are more apoptotic. Uninfected cells and cells infected with Lp02(dot/icm intact), Lp03(dotA_−), or Lp02(Δ_sidF) and Lp02(Δ_sidF/pSidF) for 14 h were fixed. Intracellular bacteria were labeled by indirect fluorescence staining with anti-L. pneumophila antibody, and the apoptotic status of the cells was probed by TUNEL staining. Samples were scored for fragmented chromatin by counting infected cells displaying positive TUNEL signals. Similar data were obtained from more than five independent experiments, and data from one representative experiment are shown.
Fig. 2.
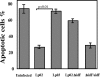
SidF is important in apoptosis resistance exhibited in L. pneumophila_-infected macrophages. Uninfected U937 or cells infected with Lp02(dot/icm intact), Lp03(dotA_−), Lp02(Δ_sidF), or Lp02(Δ_sidF/pSidF) at an MOI of 1 for 8 h were treated with 2 μM staurosporine for an additional 4 h. After fixation, bacterial vacuoles were labeled by immunostaining, and the morphology of cell nuclei was labeled with Hoechst staining. The apoptotic status of infected cells was scored by counting infected cells displaying fragmented nuclei. Data shown are averages of three independent experiments, with standard deviations.
Fig. 3.
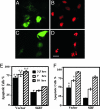
Cells expressing SidF are resistant to apoptosis induced by staurosporine. HeLa cells were transduced with adenovirus particles harboring a construct directing expression of a GFP::SidF chimera (A and B) or GFP (C and D) for 24 h before being treated with 1 μM staurosporine for 7 h. The nuclear morphology was visualized by Hoechst 33342 staining (pseudocolored in red for ease in distinguishing) (B and D). (Scale bar: 20 μm.) (E and F) Kinetic analysis of apoptotic status of the HeLa cells (E) and U937 cells (F). Transduced cells treated with 1 μM staurosporine for 3.5, 7, and 10 h were fixed, stained, and scored for the rates of apoptosis. Data shown are one representative of at least three independent experiments performed in triplicate, with at least 350 cells inspected for each sample. ∗, P < 0.05; ∗∗, P < 0.01.
Fig. 4.
SidF interacts with BNIP3 and Bcl-rambo. (A) Interactions between SidF and Bcl2-rambo/BNIP3 in yeast two-hybrid assay. Yeast strains harboring the indicated constructs were streaked on Leu− and Trp− synthetic medium to select for plasmids (on the left) or on Leu−, Trp−, Ade−, and His− medium to examine the interactions (on the right). The two SidF deletion mutants tested were SidFΔN1, SidF250–912 and SidFΔN2, SidF650–912. (B) SidF forms complexes with BNIP3. Cell lysates of 293T cells transfected with the indicated plasmid combinations were analyzed either directly or after immunoprecipitation by immunoblots with an anti-FLAG antibody. Relevant molecular mass markers are shown on the left in kilodaltons. (C) SidF in total cell lysates was detected by anti-SidF antibody. (D–F) SidF interacts with Bcl-rambo in mammalian cells. (D) 293T cells were transfected with the indicated plasmid combinations, and cell lysates were used for immunoprecipitation. Flag-Bcl-rambo was detected with an anti-FLAG antibody. (E) SidF forms a complex with endogenous Bcl-rambo. SidF was probed in immunoprecipitates obtained with anti-Bcl-rambo antibody from cells transfected with shown plasmid combinations. Note that more SidF was detected in precipitates of cells transfected with a Bcl-rambo-expressing plasmid. (F) SidF and Bcl-rambo form a complex in cells infected by a Dot/Icm-competent strain overexpressing SidF. Lysates of U937 cells infected with wild-type (lane 1), dotA−(pSidF) (lane 2), the _sidF_− mutant (lane 3), or ΔsidF(pSidF) (lane 4) for 6 h were subjected to immunoprecipitation with anti-Bcl-rambo antibody, and the presence of SidF/Bcl-rambo complexes was probed with anti-SidF antibody. (G and H) SidF directly binds Bcl-rambo. (G) Purification of GST-Bcl-rambo. Relevant molecular mass markers are shown on the left in kDa. (H) Binding between Bcl-rambo and SidF. Glutathione beads or beads coated with GST or GST-Bcl-rambo were incubated with SidF for 4 h at 4°C. After extensive washing, bound proteins were eluted with SDS loading buffer, resolved by SDS/PAGE, and probed with anti-SidF antibody (Lower). Ten percent of input proteins were detected by Coomassie bright blue staining (Upper).
Fig. 5.
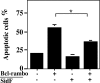
SidF inhibits apoptosis induced by Bcl-rambo. MCF-7 cells were transfected with the indicated plasmid combinations for 24 h, and fixed cells were stained with Hoechst 33342 and scored microscopically for apoptotic nuclei. Data shown are from three independent experiments performed in triplicate; at least 200 transfected cells were scored for each sample. Cells transfected with both plasmids were identified by a GFP signal carried on the vector used to express SidF. ∗, P < 0.05.
Fig. 6.
The C-terminal portion of SidF is important for its activity. (A and B) Interactions between Bcl-rambo and SidF mutants. 293T cells were transfected with combinations of plasmids expressing Flag-Bcl-rambo and individual mutants. Precipitates were prepared with anti-SidF antibody (A) or with anti-Bcl-rambo antibody (B). The presence of target proteins was detected with appropriate antibodies. In each case, 5% of the lysate used for coimmunoprecipitation was probed for levels of input proteins in total cell lysates. (C) Inhibition of Bcl-rambo activity by SidF and its derivatives. MCF-7 cells were transfected to express SidF and its derivatives along with Bcl-rambo (shaded bars) or the vector (open bars). Twenty-four hours after transfection, cells were fixed and stained with Hoechst 33342 and apoptotic rates were scored. Data shown are from three independent experiments, with standard deviations. ∗, P < 0.05; ∗∗, P < 0.01.
Similar articles
- Legionella pneumophila Strain 130b Evades Macrophage Cell Death Independent of the Effector SidF in the Absence of Flagellin.
Speir M, Vogrin A, Seidi A, Abraham G, Hunot S, Han Q, Dorn GW 2nd, Masters SL, Flavell RA, Vince JE, Naderer T. Speir M, et al. Front Cell Infect Microbiol. 2017 Feb 16;7:35. doi: 10.3389/fcimb.2017.00035. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28261564 Free PMC article. - Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila.
Abu-Zant A, Jones S, Asare R, Suttles J, Price C, Graham J, Kwaik YA. Abu-Zant A, et al. Cell Microbiol. 2007 Jan;9(1):246-64. doi: 10.1111/j.1462-5822.2006.00785.x. Epub 2006 Aug 15. Cell Microbiol. 2007. PMID: 16911566 - Incomplete activation of macrophage apoptosis during intracellular replication of Legionella pneumophila.
Abu-Zant A, Santic M, Molmeret M, Jones S, Helbig J, Abu Kwaik Y. Abu-Zant A, et al. Infect Immun. 2005 Sep;73(9):5339-49. doi: 10.1128/IAI.73.9.5339-5349.2005. Infect Immun. 2005. PMID: 16113249 Free PMC article. - Manipulation of host vesicular trafficking and innate immune defence by Legionella Dot/Icm effectors.
Ge J, Shao F. Ge J, et al. Cell Microbiol. 2011 Dec;13(12):1870-80. doi: 10.1111/j.1462-5822.2011.01710.x. Epub 2011 Nov 10. Cell Microbiol. 2011. PMID: 21981078 Review. - Autophagy Evasion and Endoplasmic Reticulum Subversion: The Yin and Yang of Legionella Intracellular Infection.
Sherwood RK, Roy CR. Sherwood RK, et al. Annu Rev Microbiol. 2016 Sep 8;70:413-33. doi: 10.1146/annurev-micro-102215-095557. Annu Rev Microbiol. 2016. PMID: 27607556 Review.
Cited by
- Legionella pneumophila regulates host cell motility by targeting Phldb2 with a 14-3-3ζ-dependent protease effector.
Song L, Luo J, Wang H, Huang D, Tan Y, Liu Y, Wang Y, Yu K, Zhang Y, Liu X, Li D, Luo ZQ. Song L, et al. Elife. 2022 Feb 17;11:e73220. doi: 10.7554/eLife.73220. Elife. 2022. PMID: 35175192 Free PMC article. - Adh enhances Actinobacillus pleuropneumoniae pathogenicity by binding to OR5M11 and activating p38 which induces apoptosis of PAMs and IL-8 release.
Wang L, Qin W, Zhang J, Bao C, Zhang H, Che Y, Sun C, Gu J, Feng X, Du C, Han W, Richard PL, Lei L. Wang L, et al. Sci Rep. 2016 Apr 5;6:24058. doi: 10.1038/srep24058. Sci Rep. 2016. PMID: 27046446 Free PMC article. - Using proteomics to identify host cell interaction partners for VgrG and IglJ.
Proksova M, Rehulkova H, Rehulka P, Lays C, Lenco J, Stulik J. Proksova M, et al. Sci Rep. 2020 Sep 3;10(1):14612. doi: 10.1038/s41598-020-71641-3. Sci Rep. 2020. PMID: 32884055 Free PMC article. - Molecular pathogenesis of infections caused by Legionella pneumophila.
Newton HJ, Ang DK, van Driel IR, Hartland EL. Newton HJ, et al. Clin Microbiol Rev. 2010 Apr;23(2):274-98. doi: 10.1128/CMR.00052-09. Clin Microbiol Rev. 2010. PMID: 20375353 Free PMC article. Review. - Proteomic analysis of growth phase-dependent expression of Legionella pneumophila proteins which involves regulation of bacterial virulence traits.
Hayashi T, Nakamichi M, Naitou H, Ohashi N, Imai Y, Miyake M. Hayashi T, et al. PLoS One. 2010 Jul 22;5(7):e11718. doi: 10.1371/journal.pone.0011718. PLoS One. 2010. PMID: 20661449 Free PMC article.
References
- Creagh EM, Conroy H, Martin SJ. Immunol Rev. 2003;193:10–21. - PubMed
- Bruggemann H, Cazalet C, Buchrieser C. Curr Opin Microbiol. 2006;9:86–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AI069344/AI/NIAID NIH HHS/United States
- R01 AI069344/AI/NIAID NIH HHS/United States
- R01 GM065260/GM/NIGMS NIH HHS/United States
- K01 CA098092/CA/NCI NIH HHS/United States
- R01GM065260/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases