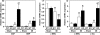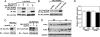Loss of muscle-specific RING-finger 3 predisposes the heart to cardiac rupture after myocardial infarction - PubMed (original) (raw)
Loss of muscle-specific RING-finger 3 predisposes the heart to cardiac rupture after myocardial infarction
Jens Fielitz et al. Proc Natl Acad Sci U S A. 2007.
Abstract
RING-finger proteins commonly function as ubiquitin ligases that mediate protein degradation by the ubiquitin-proteasome pathway. Muscle-specific RING-finger (MuRF) proteins are striated muscle-restricted components of the sarcomere that are thought to possess ubiquitin ligase activity. We show that mice lacking MuRF3 display normal cardiac function but are prone to cardiac rupture after acute myocardial infarction. Cardiac rupture is preceded by left ventricular dilation and a severe decrease in cardiac contractility accompanied by myocyte degeneration. Yeast two-hybrid assays revealed four-and-a-half LIM domain (FHL2) and gamma-filamin proteins as MuRF3 interaction partners, and biochemical analyses showed these proteins to be targets for degradation by MuRF3. Accordingly, FHL2 and gamma-filamin accumulated to abnormal levels in the hearts of mice lacking MuRF3. These findings reveal an important role of MuRF3 in maintaining cardiac integrity and function after acute myocardial infarction and suggest that turnover of FHL2 and gamma-filamin contributes to this cardioprotective function of MuRF3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
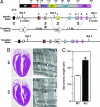
Fig. 1.
Gene targeting and generation of MuRF3 knockout mice. (A) Domains of mouse MuRF3 protein are shown at the top. RF, RING finger; MFC, MuRF family conserved domain; cc, coiled-coil; ARR, acidic-rich region. Amino acids are shown above the protein structure. The targeting vector contained a 4.5-kb 5′ arm, a 1.7-kb 3′ arm, a neomycin cassette (Neo), and a thymidine kinase gene (tk). Exons 1–9 are shown in boxes. Positions of 5′ and 3′ probes and PCR primers are indicated. (B) H&E-stained sections of 8-week-old WT and _MuRF3_−/− (KO) hearts. (Right) Representative sarcomeric structures visualized by transmission electron microscopy. (×6,000 magnification; scale bar, 2 μm.) (C) The distance between sarcomeric Z-lines in cardiomyocytes from WT and _MuRF3_−/− (KO) hearts as visualized by electron microscopy. Arrows indicate Z-lines. ∗, P < 0.01 vs. WT.
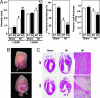
Fig. 2.
Analyses of _MuRF3_−/− hearts. (A) Echocardiographic measurements of WT sham (n = 10), WT MI (n = 12), _MuRF3_−/− (KO) sham (n = 7), and KO MI (n = 12) mice. LVESD, left ventricular end-systolic dimension; LVEDD, left ventricular end-diastolic dimension. (B) Representative _MuRF3_−/− ruptured hearts 3 days after MI. Arrow indicates site of rupture. (C) H&E-stained sections of WT and _MuRF3_−/− (KO) hearts after sham operation or MI. Arrow indicates site of rupture. (Right) High-magnification images of the periinfarct zone (marked by box in Center). ∗, P < 0.01 vs. sham; †, P < 0.01 vs. WT MI.
Fig. 3.
Expression of cardiac stress markers. Real-time RT-PCR was used to measure the expression of transcripts encoding ANF, BNP, α-MHC, β-MHC, and c-fos in the hearts of WT sham (n = 4), WT MI (n = 5), _MuRF3_−/− (KO) sham (n = 6), and KO MI (n = 6) mice. ∗, P < 0.01 vs. sham; †, P < 0.01 vs. WT MI.
Fig. 4.
Treatment of _MuRF3_−/− mice with isoproterenol reduces cardiac performance. (A) Representative H&E-stained sections of hearts from WT and _MuRF3_−/− (KO) mice treated with vehicle or isoproterenol (Iso) for 7 days. (B) Fractional shortening measured by echocardiography from WT vehicle-treated (n = 5), WT Iso-treated (n = 8), _MuRF3_−/− (KO) vehicle-treated (n = 4), and KO Iso-treated (n = 8) mice. (C) Numbers of TUNEL-positive nuclei per left ventricular section of WT vehicle-treated (n = 2), WT Iso-treated (n = 3), _MuRF3_−/− (KO) vehicle-treated (n = 2), and KO Iso-treated (n = 3) mice. n.d., none detected. ∗, P < 0.01 vs. sham; †, P < 0.01 vs. WT Iso.
Fig. 5.
MuRF3 interacts with and degrades FHL2 and γ-filamin. C2C12 cells were cotransfected with expression plasmids encoding Myc-MuRF3 or Myc-MuRF3Δcc1 and HA-FHL2 (A) or Flag-filamin A (Flag-FLNA) or Flag-γ-filamin (Flag-FLNC) (B) and coimmunoprecipitated 24 h posttransfection by using Myc or Flag antibody and immunoblotted with HA or Myc antibody. IP, immunoprecipitated; IB, immunoblotted. (C) C2C12 cells were cotransfected with expression plasmids encoding HA-FHL2 or Flag-γ-filamin (Flag-FLNC) with or without Myc-tagged or nontagged MuRF3. Protein lysates were used in Western blot analyses with HA or Flag antibody. α-Tubulin was used as control. (D) Endogenous FHL2 and γ-filamin (FLNC) proteins were measured in extracts from hearts of three individual WT and _MuRF3_−/− (KO) mice by using FHL2 and γ-filamin-specific antibodies. GAPDH was used as a control. (E) FHL2 and γ-filamin mRNA were measured by quantitative real-time PCR using ABI TaqMan probes and normalized to GAPDH. No change in mRNA expression of FHL2 and γ-filamin was found between WT (n = 4) and _MuRF3_−/− (KO, n = 4) mice hearts.
Similar articles
- Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I.
Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. Kedar V, et al. Proc Natl Acad Sci U S A. 2004 Dec 28;101(52):18135-40. doi: 10.1073/pnas.0404341102. Epub 2004 Dec 15. Proc Natl Acad Sci U S A. 2004. PMID: 15601779 Free PMC article. - Muscle ring finger-3 protects against diabetic cardiomyopathy induced by a high fat diet.
Quintana MT, He J, Sullivan J, Grevengoed T, Schisler J, Han Y, Hill JA, Yates CC, Stansfield WE, Mapanga RF, Essop MF, Muehlbauer MJ, Newgard CB, Bain JR, Willis MS. Quintana MT, et al. BMC Endocr Disord. 2015 Jul 28;15:36. doi: 10.1186/s12902-015-0028-z. BMC Endocr Disord. 2015. PMID: 26215257 Free PMC article. - Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2.
Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S. Witt CC, et al. EMBO J. 2008 Jan 23;27(2):350-60. doi: 10.1038/sj.emboj.7601952. Epub 2007 Dec 20. EMBO J. 2008. PMID: 18157088 Free PMC article. - Build it up-Tear it down: protein quality control in the cardiac sarcomere.
Willis MS, Schisler JC, Portbury AL, Patterson C. Willis MS, et al. Cardiovasc Res. 2009 Feb 15;81(3):439-48. doi: 10.1093/cvr/cvn289. Epub 2008 Oct 29. Cardiovasc Res. 2009. PMID: 18974044 Free PMC article. Review. - Muscle LIM Protein: Master regulator of cardiac and skeletal muscle functions.
Vafiadaki E, Arvanitis DA, Sanoudou D. Vafiadaki E, et al. Gene. 2015 Jul 15;566(1):1-7. doi: 10.1016/j.gene.2015.04.077. Epub 2015 Apr 30. Gene. 2015. PMID: 25936993 Free PMC article. Review.
Cited by
- Unlocking the secrets of Cardiac development and function: the critical role of FHL2.
Jiang T, Zeng Q, Wang J. Jiang T, et al. Mol Cell Biochem. 2024 Oct 28. doi: 10.1007/s11010-024-05142-6. Online ahead of print. Mol Cell Biochem. 2024. PMID: 39466483 Review. - Metabolic remodeling in cardiac hypertrophy and heart failure with reduced ejection fraction occurs independent of transcription factor EB in mice.
Dörmann N, Hammer E, Struckmann K, Rüdebusch J, Bartels K, Wenzel K, Schulz J, Gross S, Schwanz S, Martin E, Fielitz B, Pablo Tortola C, Hahn A, Benkner A, Völker U, Felix SB, Fielitz J. Dörmann N, et al. Front Cardiovasc Med. 2024 Jan 8;10:1323760. doi: 10.3389/fcvm.2023.1323760. eCollection 2023. Front Cardiovasc Med. 2024. PMID: 38259303 Free PMC article. - Identification of hypertrophy-modulating Cullin-RING ubiquitin ligases in primary cardiomyocytes.
Fischer M, Jakab M, Hirt MN, Werner TR, Engelhardt S, Sarikas A. Fischer M, et al. Front Physiol. 2023 Mar 8;14:1134339. doi: 10.3389/fphys.2023.1134339. eCollection 2023. Front Physiol. 2023. PMID: 36969608 Free PMC article. - The Transcription Factor EB (TFEB) Sensitizes the Heart to Chronic Pressure Overload.
Wundersitz S, Pablo Tortola C, Schmidt S, Oliveira Vidal R, Kny M, Hahn A, Zanders L, Katus HA, Sauer S, Butter C, Luft FC, Müller OJ, Fielitz J. Wundersitz S, et al. Int J Mol Sci. 2022 May 25;23(11):5943. doi: 10.3390/ijms23115943. Int J Mol Sci. 2022. PMID: 35682624 Free PMC article. - The Role of Muscle Ring Finger-1 (MuRF1), MuRF2, MuRF3, and Atrogin-1 on Bone Microarchitecture In Vivo.
Suryadevara V, Krehbial CJ, Halsey D, Willis MS. Suryadevara V, et al. Cell Biochem Biophys. 2022 Jun;80(2):415-426. doi: 10.1007/s12013-022-01069-1. Epub 2022 Feb 21. Cell Biochem Biophys. 2022. PMID: 35191000
References
- Wang X, Robbins J. Circ Res. 2006;99:1315–1328. - PubMed
- Ciechanover A. Neurology. 2006;66:S7–S19. - PubMed
- Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB, Taegtmeyer H. Circulation. 2003;108:2536–2541. - PubMed
- Glass DJ. Nat Cell Biol. 2003;5:87–90. - PubMed
- Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, et al. Circulation. 2006;114:1821–1828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases