Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases - PubMed (original) (raw)
Comparative Study
. 2007 Feb 27;104(9):3055-60.
doi: 10.1073/pnas.0611478104. Epub 2007 Feb 20.
Affiliations
- PMID: 17360608
- PMCID: PMC1802009
- DOI: 10.1073/pnas.0611478104
Comparative Study
Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases
Erica A Moehle et al. Proc Natl Acad Sci U S A. 2007.
Erratum in
- Proc Natl Acad Sci U S A. 2007 Apr 3;104(14):6090. Moehle, E A [corrected to Moehle, Erica A]; Rock, J M [corrected to Rock, Jeremy M]; Lee, Y L [corrected to Lee, Ya-Li]; Jouvenot, Y [corrected to Jouvenot, Yann]; Dekelver, R C [corrected to DeKelver, Russell C]; Gregory, P D [corrected to Gregory, Philip
Abstract
Efficient incorporation of novel DNA sequences into a specific site in the genome of living human cells remains a challenge despite its potential utility to genetic medicine, biotechnology, and basic research. We find that a precisely placed double-strand break induced by engineered zinc finger nucleases (ZFNs) can stimulate integration of long DNA stretches into a predetermined genomic location, resulting in high-efficiency site-specific gene addition. Using an extrachromosomal DNA donor carrying a 12-bp tag, a 900-bp ORF, or a 1.5-kb promoter-transcription unit flanked by locus-specific homology arms, we find targeted integration frequencies of 15%, 6%, and 5%, respectively, within 72 h of treatment, and with no selection for the desired event. Importantly, we find that the integration event occurs in a homology-directed manner and leads to the accurate reconstruction of the donor-specified genotype at the endogenous chromosomal locus, and hence presumably results from synthesis-dependent strand annealing repair of the break using the donor DNA as a template. This site-specific gene addition occurs with no measurable increase in the rate of random integration. Remarkably, we also find that ZFNs can drive the addition of an 8-kb sequence carrying three distinct promoter-transcription units into an endogenous locus at a frequency of 6%, also in the absence of any selection. These data reveal the surprising versatility of the specialized polymerase machinery involved in double-strand break repair, illuminate a powerful approach to mammalian cell engineering, and open the possibility of ZFN-driven gene addition therapy for human genetic disease.
Conflict of interest statement
Conflict of interest statement: C.O.P. is chair of the Scientific Advisory Board for Sangamo BioSciences, Inc. E.A.M., J.M.R., Y.-L.L., Y.J., R.C.D., P.D.G., F.D.U., and M.C.H. are full-time employees of Sangamo BioSciences, Inc.
Figures
Fig. 1.
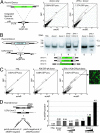
A ZFN-induced DSB leads to efficient, homology-based tag transfer into a native chromosomal locus. (A) Experimental outline and a schematic of the process whereby a ZFN-induced DSB is repaired by using an extrachromosomal donor as a template. (B) PCR-based measurements of ZFN-driven tag integration frequency into the IL2Rγ locus in K562 cells. Cells were left untransfected (first lane, “neg.” for negative control) or were transfected with an expression cassette for ZFNs that induce a DSB at exon 5 of IL2Rγ (16) (second lane), and donor plasmids carrying a 12-bp tag flanked by 750-bp homology arms, in the absence (third lane) and presence (fourth lane) of the IL2Rγ ZFNs. Genomic DNA was extracted 72 h later. The IL2Rγ locus was amplified by 20 cycles of PCR in the presence of radiolabeled dNTPs by using primers that hybridize to the chromosome outside of the donor homology arms, and the PCR products were digested with StuI, resolved by 10% PAGE, and autoradiographed. The percentage of StuI-sensitive DNA is indicated below the fourth lane. (C) Sequence analysis of ZFN-edited chromatids. The primary DNA sequence, and the amino acid sequence it encodes, of exon 5 of the human IL2Rγ gene, along with the target sites of the designed ZFNs, are indicated. The central portion of the donor sequence, along with the tag, is shown below. A representative chromatogram of the DNA sequence of one of the chromatids obtained from sample 4 (in B) is provided, showing the chromosomal sequence to be altered precisely in the manner specified by the donor, i.e., by copy-pasting of codons for four new amino acids in-frame with the endogenous ORF. Note that an additional silent SNP (Pro229 CCA→CCT), introduced for cloning purposes, is also transferred from the donor.
Fig. 2.
ZFN-driven targeted integration of a series of progressively larger DNA sequences into an endogenous locus. (A Left) A schematic of a chromosomal reporter construct in HEK293 cells that contains the recognition site for two ZFNs (gray box) and a donor molecule that carries the GFP ORF (green rectangle) flanked by homology arms. The percentage of GFP-positive HEK293 cells was measured by FACS (Center and Right) and is indicated in each panel. (B) PCR-based measurements of ZFN-driven integration frequency into the IL2Rγ locus in K562 cells. Cells were left untransfected (lane 1) or were transfected with an expression cassette for ZFNs that induce a DSB at exon 5 of IL2Rγ (16) (lane 2), and donor plasmids carrying the indicated inserts flanked by 750-bp homology arms, in the absence (lanes 3 and 6) and presence (lanes 4, 5, and 7) of the IL2Rγ ZFNs. The donor DNAs tested were as follows: a 900-bp GFP ORF (lane 4) or the same ORF followed by a polyA sequence (lane 5) and an autonomous expression cassette (human phosphoglycerokinase promoter–GFP–polyA; lane 7). Genomic DNA was amplified by using primers outside the donor homology arms, and the level of targeted integration was determined by PAGE and autoradiography (the integrant-carrying chromosome migrates above the wild-type one). The integration frequency is indicated for each panel. Note that the autoradiograph for lanes 6 and 7 was generated in an experiment distinct from that for lanes 1–5. (C) Functional measurement of targeted integration frequency. The percentage of GFP-positive cells was measured by FACS in K562 cells transfected with an IL2Rγ donor molecule carrying an autonomous GFP expression cassette (Center; see donor schematic at the bottom B), transfected with this donor and IL2Rγ-specific ZFNs (Right) or untreated control cells (Left), and is shown within each panel. All measurements were taken 3 weeks after transfection to permit decay of expression from the donor episome. A fluorescence micrograph of an aliquot of the GFP-positive cells from Right is also shown. (D) FACS-based measurement of the rate of plasmid DNA random integration. (Left) The plasmid donor construct (a tag-interrupted homology stretch flanked by an autonomous expression cassette for a cell surface marker, ΔNGFR). Cell phenotypes expected from a targeted (lower left) or random (lower right) integration event are shown. (Right) FACS data from an experiment in which K562 cells were treated with only the donor molecule, the donor molecule together with the ZFN expression cassette, or the donor molecule and an increasing concentration of etoposide. The percentage of cells positive for the ΔNGFR marker (as measured by FACS after sufficient cell passaging to allow for donor DNA decay) in each sample is indicated.
Fig. 3.
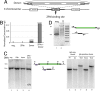
ZFN-driven integration of an ≈8-kb DNA sequence encoding multiple transgenes into an endogenous locus. (A) A schematic representation indicating the design of the donor DNA plasmid containing the 750-bp homologous flanking sequence of the IL2Rγ exon5 region and the three promoter-transcription units (line arrows). The site of cleavage of the ZFNs for IL2Rγ is indicated. (B) ELISA for IgG in culture medium. Medium was collected from K562 cells after the indicated treatments, and levels of secreted IgG were measured by performing an ELISA with an antibody for the heavy and light chains of IgG. IgG concentration is expressed in nanograms per milliliter per cell. (C) Southern blot-based measurement of targeted integration frequency. (Left) The same genomic DNA preparations as in B were digested with HindIII, and Southern blotting was performed with a fragment of the IL2Rγ locus that lies adjacent to the left donor homology arm. The locus maps in Center indicate the restriction map of a wild-type chromosome (bottom, 3.8 kb) and a chromosome carrying the integrated transgene (top, 4.9 kb), and the probe used is indicated with an open box. H, HindIII site. (Right) Southern blot analysis of cell clones (see
SI Fig. 6_C_
for their genotypes). Lanes 5 and 6, control clones that do not secrete IgG into the medium; lanes 7 and 9, cells that secrete IgG into the medium and appear to carry the insert at IL2Rγ as gauged by PCR (
SI Fig. 6_C_
); lane 8, cells that secrete IgG into the medium and do not appear to carry the insert at IL2Rγ as gauged by PCR (
SI Fig. 6_C_
). Genomic DNA from single-cell-derived clones was isolated and digested with HindIII and probed as in Left. (D) PCR across the integrated expression cassette. PCR was performed on genomic DNA isolated from clones either negative (lane 1) or positive (lane 2) for transgene integration after transfection with the ZFNs and the donor plasmid, using a high-fidelity polymerase and primers outside of the donor homology arms (arrows in locus map).
Fig. 4.
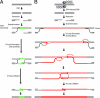
ZFN-driven repair of endogenous DSBs and of ZFN-induced breaks followed by homology-directed targeted integration. (A) SDSA-based HDR of an endogenous break (32). After a DSB a single-stranded chromosomal tail invades a sister chromatid, and after DNA synthesis of a short stretch the D loop collapses the newly synthesized DNA. (B) Homology-directed targeted integration after a ZFN-induced break and SDSA-based transfer of genetic information into the break, a model most consistent with the data presented in the current work and evidence in the literature. After a ZFN-induced break, the single-stranded chromosome end homes into the homology arm carried by the donor (this process is unimpeded by the presence of the insert because the latter is located precisely at the position corresponding to the break and hence remains “invisible” to the homology search mechanism). Synthesis then proceeds across at least 50% of the insert length, with the newly synthesized single-stranded DNA trailing the D loop. Irrespective of whether the two broken chromosomal ends use the same (as shown) or two different donor molecules as templates, once synthesis in each D loop has proceeded long enough for the two DNA stretches to overlap, the newly synthesized DNA molecules can leave the D loop, anneal to each other, and then use each other as templates to restore an intact chromatid, now carrying the donor-specified transgene at the chromosome.
Similar articles
- Gene targeting using zinc finger nucleases.
Porteus MH, Carroll D. Porteus MH, et al. Nat Biotechnol. 2005 Aug;23(8):967-73. doi: 10.1038/nbt1125. Nat Biotechnol. 2005. PMID: 16082368 Review. - Origins of Programmable Nucleases for Genome Engineering.
Chandrasegaran S, Carroll D. Chandrasegaran S, et al. J Mol Biol. 2016 Feb 27;428(5 Pt B):963-89. doi: 10.1016/j.jmb.2015.10.014. Epub 2015 Oct 23. J Mol Biol. 2016. PMID: 26506267 Free PMC article. Review. - Targeted gene addition to a predetermined site in the human genome using a ZFN-based nicking enzyme.
Wang J, Friedman G, Doyon Y, Wang NS, Li CJ, Miller JC, Hua KL, Yan JJ, Babiarz JE, Gregory PD, Holmes MC. Wang J, et al. Genome Res. 2012 Jul;22(7):1316-26. doi: 10.1101/gr.122879.111. Epub 2012 Mar 20. Genome Res. 2012. PMID: 22434427 Free PMC article. - Zinc finger nucleases: custom-designed molecular scissors for genome engineering of plant and mammalian cells.
Durai S, Mani M, Kandavelou K, Wu J, Porteus MH, Chandrasegaran S. Durai S, et al. Nucleic Acids Res. 2005 Oct 26;33(18):5978-90. doi: 10.1093/nar/gki912. Print 2005. Nucleic Acids Res. 2005. PMID: 16251401 Free PMC article. Review. - Targeted genome editing in pluripotent stem cells using zinc-finger nucleases.
Bobis-Wozowicz S, Osiak A, Rahman SH, Cathomen T. Bobis-Wozowicz S, et al. Methods. 2011 Apr;53(4):339-46. doi: 10.1016/j.ymeth.2010.12.019. Epub 2010 Dec 23. Methods. 2011. PMID: 21185378
Cited by
- Identification of Site in the UTY Gene as Safe Harbor Locus on the Y Chromosome of Pig.
Chen X, Yang G, Ji P, Liu G, Zhang L. Chen X, et al. Genes (Basel). 2024 Aug 1;15(8):1005. doi: 10.3390/genes15081005. Genes (Basel). 2024. PMID: 39202365 Free PMC article. - Gene therapy for retinal diseases: From genetics to treatment.
Khaparde A, Mathias GP, Poornachandra B, Thirumalesh MB, Shetty R, Ghosh A. Khaparde A, et al. Indian J Ophthalmol. 2024 Aug 1;72(8):1091-1101. doi: 10.4103/IJO.IJO_2902_23. Epub 2024 Jul 29. Indian J Ophthalmol. 2024. PMID: 39078952 Free PMC article. Review. - Advance trends in targeting homology-directed repair for accurate gene editing: An inclusive review of small molecules and modified CRISPR-Cas9 systems.
Shams F, Bayat H, Mohammadian O, Mahboudi S, Vahidnezhad H, Soosanabadi M, Rahimpour A. Shams F, et al. Bioimpacts. 2022;12(4):371-391. doi: 10.34172/bi.2022.23871. Epub 2022 Jun 22. Bioimpacts. 2022. PMID: 35975201 Free PMC article. Review. - Transcription-coupled donor DNA expression increases homologous recombination for efficient genome editing.
Gao K, Zhang X, Zhang Z, Wu X, Guo Y, Fu P, Sun A, Peng J, Zheng J, Yu P, Wang T, Ye Q, Jiang J, Wang H, Lin CP, Gao G. Gao K, et al. Nucleic Acids Res. 2022 Oct 28;50(19):e109. doi: 10.1093/nar/gkac676. Nucleic Acids Res. 2022. PMID: 35929067 Free PMC article. - Gene-Edited Cell Models to Study Chronic Wasting Disease.
Thapa S, Marrero Winkens C, Tahir W, Arifin MI, Gilch S, Schatzl HM. Thapa S, et al. Viruses. 2022 Mar 15;14(3):609. doi: 10.3390/v14030609. Viruses. 2022. PMID: 35337016 Free PMC article. Review.
References
- Klug A. Ann NY Acad Sci. 1995;758:143–160. - PubMed
- Tupler R, Perini G, Green MR. Nature. 2001;409:832–833. - PubMed
- Pavletich NP, Pabo CO. Science. 1991;252:809–817. - PubMed
- Choo Y, Sanchez-Garcia I, Klug A. Nature. 1994;372:642–645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources