Circadian variation of blood pressure and the vascular response to asynchronous stress - PubMed (original) (raw)
Comparative Study
. 2007 Feb 27;104(9):3450-5.
doi: 10.1073/pnas.0611680104. Epub 2007 Feb 20.
Affiliations
- PMID: 17360665
- PMCID: PMC1802007
- DOI: 10.1073/pnas.0611680104
Comparative Study
Circadian variation of blood pressure and the vascular response to asynchronous stress
Anne M Curtis et al. Proc Natl Acad Sci U S A. 2007.
Abstract
The diurnal variation in the incidence of myocardial infarction and stroke may reflect an influence of the molecular clock and/or the time dependence of exposure to environmental stress. The circadian variation in blood pressure and heart rate is disrupted in mice, Bmal1(-/-), Clock(mut), and Npas2(mut), in which core clock genes are deleted or mutated. Although Bmal1 deletion abolishes the 24-h frequency in cardiovascular rhythms, a shorter ultradian rhythm remains. Sympathoadrenal function is disrupted in these mice, which reflects control of enzymes relevant to both synthesis (phenylethanolamine N-methyl transferase) and disposition (monoamine oxidase B and catechol-O-methyl transferase) of catecholamines by the clock. Both timing and disruption or mutation of clock genes modulate the magnitude of both the sympathoadrenal and pressor but not the adrenocortical response to stress. Despite diurnal variation of catecholamines and corticosteroids, they are regulated differentially by the molecular clock. Furthermore, the clock may influence the time-dependent incidence of cardiovascular events by controlling the integration of selective asynchronous stress responses with an underlying circadian rhythm in cardiovascular function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
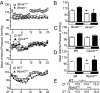
Fig. 1.
BMAL1 and CLOCK and NPAS2 differ in severity of BP variation and maintenance according to the severity of the genotype. (A) MAP telemetry recordings averaged each hour from three 24-h periods in WT, _Bmal1_−/−, Clockmut, and Npas2mut mice. MAP recordings were significantly different (Kruskal–Wallis test) for _Bmal1_−/− (light phase, P < 0.05; dark phase, P < 0.0001) and Npas2mut mice (both light and dark phase. P < 0.0001). Clockmut mice were significantly different only during the light phase (P < 0.05). MAP recordings taken separately during light and dark phases in Bmal1+/+ vs. _Bmal1_−/− (B) (n = 8; ∗, P < 0.05; †, P < 0.001), WT vs. Clockmut (C) (n = 4–8) WT vs. Npas2mut mice (D) (n = 8; ∗, P < 0.05), and delayed acrophase (time of peak) of MAP rhythm in Npas_2_mut vs. WT (D) (n = 8, P < 0.05). White boxes denote lights on, and dark boxes denote lights off.
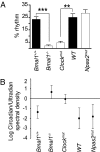
Fig. 2.
Circadian but not ultradian rhythmicity depends on BMAL1 and CLOCK. Twenty-four-hour period harmonic (A) and log ratio of circadian and ultradian spectal values (B) were calculated for Bmal1+/+, _Bmal1_−/[supi]−, Clockmut, WT, and Npas2mut mice.
Fig. 3.
Pnmt, maoB, and comt are under clock control in hearts and adrenals. mRNA was analyzed by real-time PCR at ZT2, ZT6, ZT14, and ZT18 for pnmt expression (A) and maoB expression (D) in hearts of Bmal1+/+ vs. _Bmal1_−/− mice (n = 4–8; ∗, P < 0.01) pnmt expression (B) and maoB expression (E) in adrenals of Bmal1+/+ vs. _Bmal1_−/− mice (n = 4–8; ∗, P < 0.01) maoB and pnmt expression in WT and Npas2mut (C) (n = 4–5; ∗, P < 0.05), and comt and pnmt expression in WT and Clockmut (F) (n = 4; ∗, P < 0.05).
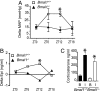
Fig. 4.
The circadian clock modulates pressor and hormonal but not adrenocortical responses to stress. (A) Telemetry recordings of MAP were taken at baseline and after restraint within each mouse. Delta MAP values were calculated for Bmal1+/+ and _Bmal1_−/− at ZT0, ZT6, ZT12, and ZT18, (n = 4–8; ANOVA, ∗, P < 0.05). (B) Serum was collected by retroorbital bleed from naïve (baseline) Bmal1+/+ and _Bmal1_−/− mice and naïve Bmal1+/+ and _Bmal1_−/− mice after 15-min restraint at ZT0, ZT6, ZT12, and ZT18, and delta Epi values were calculated for both Bmal1+/+ and _Bmal1_−/− responses (n, 4–8; ∗, P < 0.05). (C) Glucocorticoid analysis from serum collected as in B from animals at ZT12 at baseline (B) or immobilized (I).
Similar articles
- Genetic components of the circadian clock regulate thrombogenesis in vivo.
Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. Westgate EJ, et al. Circulation. 2008 Apr 22;117(16):2087-95. doi: 10.1161/CIRCULATIONAHA.107.739227. Epub 2008 Apr 14. Circulation. 2008. PMID: 18413500 - Dual role of the CLOCK/BMAL1 circadian complex in transcriptional regulation.
Kondratov RV, Shamanna RK, Kondratova AA, Gorbacheva VY, Antoch MP. Kondratov RV, et al. FASEB J. 2006 Mar;20(3):530-2. doi: 10.1096/fj.05-5321fje. Epub 2006 Jan 25. FASEB J. 2006. PMID: 16507766 - Postnatal ontogenesis of molecular clock in mouse striatum.
Cai Y, Liu S, Li N, Xu S, Zhang Y, Chan P. Cai Y, et al. Brain Res. 2009 Apr 6;1264:33-8. doi: 10.1016/j.brainres.2009.01.003. Epub 2009 Jan 10. Brain Res. 2009. PMID: 19171124 - [Synchronization and genetic redundancy in circadian clocks].
Dardente H. Dardente H. Med Sci (Paris). 2008 Mar;24(3):270-6. doi: 10.1051/medsci/2008243270. Med Sci (Paris). 2008. PMID: 18334175 Review. French. - Role for the Clock gene in bipolar disorder.
McClung CA. McClung CA. Cold Spring Harb Symp Quant Biol. 2007;72:637-44. doi: 10.1101/sqb.2007.72.031. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419323 Review.
Cited by
- Inhibition of G0/G1 Switch 2 Ameliorates Renal Inflammation in Chronic Kidney Disease.
Matsunaga N, Ikeda E, Kakimoto K, Watanabe M, Shindo N, Tsuruta A, Ikeyama H, Hamamura K, Higashi K, Yamashita T, Kondo H, Yoshida Y, Matsuda M, Ogino T, Tokushige K, Itcho K, Furuichi Y, Nakao T, Yasuda K, Doi A, Amamoto T, Aramaki H, Tsuda M, Inoue K, Ojida A, Koyanagi S, Ohdo S. Matsunaga N, et al. EBioMedicine. 2016 Nov;13:262-273. doi: 10.1016/j.ebiom.2016.10.008. Epub 2016 Oct 6. EBioMedicine. 2016. PMID: 27745900 Free PMC article. - Effects of stressor controllability on diurnal physiological rhythms.
Thompson RS, Christianson JP, Maslanik TM, Maier SF, Greenwood BN, Fleshner M. Thompson RS, et al. Physiol Behav. 2013 Mar 15;112-113:32-9. doi: 10.1016/j.physbeh.2013.02.009. Epub 2013 Feb 27. Physiol Behav. 2013. PMID: 23454291 Free PMC article. - A wheel of time: the circadian clock, nuclear receptors, and physiology.
Yang X. Yang X. Genes Dev. 2010 Apr 15;24(8):741-7. doi: 10.1101/gad.1920710. Genes Dev. 2010. PMID: 20395363 Free PMC article. - Understanding Quantitative Circadian Regulations Are Crucial Towards Advancing Chronotherapy.
Chowdhury D, Wang C, Lu AP, Zhu HL. Chowdhury D, et al. Cells. 2019 Aug 13;8(8):883. doi: 10.3390/cells8080883. Cells. 2019. PMID: 31412622 Free PMC article. Review. - Chronobiological Influence Over Cardiovascular Function: The Good, the Bad, and the Ugly.
Rana S, Prabhu SD, Young ME. Rana S, et al. Circ Res. 2020 Jan 17;126(2):258-279. doi: 10.1161/CIRCRESAHA.119.313349. Epub 2020 Jan 16. Circ Res. 2020. PMID: 31944922 Free PMC article. Review.
References
- Muller JE. Am J Hypertens. 1999;12:35S–42S. - PubMed
- Manfredini R, Boari B, Smolensky MH, Salmi R, Gallerani M, Guerzoni F, Guerra V, Maria Malagoni A, Manfredini F. Chronobiol Int. 2005;22:1121–1135. - PubMed
- Hastings MH, Reddy AB, Maywood ES. Nat Rev Neurosci. 2003;4:649–661. - PubMed
- Weber MA, Fodera SM. Rev Cardiovasc Med. 2004;5:148–155. - PubMed
- Ralph MR, Foster RG, Davis FC, Menaker M. Science. 1990;247:975–978. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases