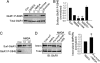Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit - PubMed (original) (raw)
Comparative Study
. 2007 Feb 27;104(9):3579-84.
doi: 10.1073/pnas.0611698104. Epub 2007 Feb 21.
Affiliations
- PMID: 17360685
- PMCID: PMC1805611
- DOI: 10.1073/pnas.0611698104
Comparative Study
Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit
Heng-Ye Man et al. Proc Natl Acad Sci U S A. 2007.
Abstract
alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors mediate the majority of excitatory synaptic transmission in the brain. Recent studies have shown that activation of PKA regulates the membrane trafficking of the AMPA receptor Glu receptor 1 (GluR1) subunit, but the role of direct phosphorylation of GluR1 in regulating receptor redistribution is not clear. Here we show that phosphorylation of the GluR1 subunit on serine 845 by PKA is required for PKA-induced increases in AMPA receptor cell-surface expression because it promotes receptor insertion and decreases receptor endocytosis. Furthermore, dephosphorylation of GluR1 serine 845 triggers NMDA-induced AMPA receptor internalization. These findings strongly suggest that dynamic changes in direct phosphorylation of GluR1 by PKA are crucial in the modulation of AMPA receptor trafficking and synaptic plasticity.
Conflict of interest statement
Conflict of interest statement: Under a licensing agreement between Upstate Group, Inc., and The Johns Hopkins University, R.L.H. is entitled to a share of royalties received by the university on sales of products described in this article. R.L.H. is a paid consultant to Upstate Group, Inc. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies.
Figures
Fig. 1.
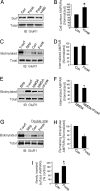
PKA activation increases AMPA receptor cell-surface expression in cultured cortical neurons. (A) Cell-surface biotinylation shows an increase in AMPA receptor surface expression (Surf) by using forskolin treatment (20 μM forskolin plus 50 μM 3-isobutyl-1-methylxanthine) (Forsk) compared with control (Con). This effect was abolished with PKA-specific inhibitor H89 (Forsk+H89). No changes were found in the total AMPA receptor protein amount (Total). (B) Densitometric quantitation of Western blots on AMPA receptor surface biotinylation. Data represent means ± SE (n = 4; ∗, P < 0.05 relative to control, _t_ test). (_C_ and _D_) Forskolin treatments show no effect on AMPA receptor constitutive internalization. Surface biotinylation at 4°C without stripping produced total surface receptors (T-surf), and stripping immediately after biotinylation showed low background (Strip and Con) (_n_ = 3; _P_ > 0.05, t test). (E and F) PKA inhibits NMDA-induced AMPA receptor internalization. Surface-biotinylated neurons were incubated at 37°C in the presence of 30 μM NMDA, with (NMDA+Forsk) or without (NMDA) forskolin treatment, for 15 min to induce receptor endocytosis (n = 4; ∗, P < 0.05, t test). (G and H) Surface biotinylation-based receptor insertion assays. Forskolin treatment decreased the remaining endocytosed receptor amount by using double strips (n = 2), indicating a facilitated receptor reinsertion. (I) AMPA receptor surface insertion by colorimetric assays. Surface AMPA receptors were first blocked with an anti-GluR1 N terminus antibody and a cold (nonconjugated) secondary antibody. After incubation at 37°C with (Forsk) or without (Con) forskolin treatment, newly inserted surface receptors were detected by using a second round of antibody labeling (n = 8; ∗, P < 0.05 compared with control, t test).
Fig. 2.
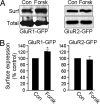
PKA-dependent increase in AMPA receptor cell-surface expression is subunit-specific. (A) Surface biotinylation assays in transfected HEK cells. Forskolin treatment for 15 min increased the AMPA receptor subunit surface expression (Surf) in HEK cells expressing GluR1-GFP (Left) but not in those expressing GluR2-GFP (Right). (B) Quantitation of cell-surface expression of AMPA receptor subunits in HEK cells. Forskolin treatment (Forsk) increased GluR1-GFP surface expression (n = 3; ∗, P < 0.05, _t_ test) (_Left_) but did not change the abundance of cell-surface GluR2-GFP (_n_ = 3; _P_ > 0.05) (Right). Con, control.
Fig. 3.
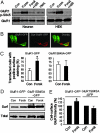
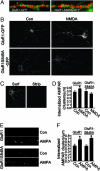
Phosphorylation of GluR1S845 is required in PKA activity-dependent regulation of AMPA receptor surface expression. (A) PKA phosphorylates GluR1S845 in both cortical neurons and GluR1-expressing HEK cells. Cultured cortical neurons (Left) or HEK cells transiently transfected with GluR1-GFP (Right) were treated with forskolin (Forsk) for 10 min, and GluR1S845 phosphorylation was examined by using anti-phospho-GluR1S845 (GluRI p-S845) antibodies. Compared with controls (Con), forskolin treatment greatly increased the phosphorylation level that was blocked by the PKA inhibitor H89 (Forsk+H89) (2 μM), and the PKC activator PMA (1 μM) had no effect. (B and C) PKA increases surface-GluR1 positive rate in transfected HEK cells. (B) In GluR1-GFP-expressing or GluR1S845A-GFP-expressing HEK cells, surface staining (red) revealed that a certain amount of cells had no visible surface labeling. (C) PKA treatment increased the percentage of surface-positive cells in the GluR1-expressing HEK population (Left) (n = 300 transfected cells in three experiments; ∗, P < 0.05 relative to control, _t_ test) but not in cells expressing GluR1S845A (_Right_) (_n_ = 300 transfected cells in three experiments). (_D_) Surface biotinylation assays showed no effect of forskolin treatment on GluR1S845A surface expression in transfected HEK cells. (_E_) Densitometric quantitation of surface biotinylation experiments in _D_. Forskolin treatment significantly increased the surface expression of GluR1-GFP (_Left_) (_n_ = 5; ∗, _P_ < 0.05, _t_ test), but not GluR1S845A-GFP (_Right_) (_n_ = 5; _P_ > 0.05, t test).
Fig. 4.
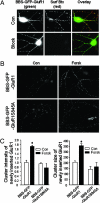
Forskolin increases GluR1 cell-surface insertion rate in an S845-dependent manner in transfected cortical neurons. (A) Cortical neurons were transfected with GluR1 subunit (green) tagged with a BBS and GFP at its extracellular N terminus (BBS-GFP-GluR1). Rhodamine-Btx surface binding assay demonstrated that BBS-GFP-GluR1 was expressed on cell surface in clusters (Con). Immediately after incubation with free Btx, rhodamine-Btx labeling showed no signal, indicating a complete block of surface GluR1 BBS sites (Block). (B) Forskolin treatment facilitated GluR1 cell-surface insertion rate (BBS-GFP-GluR1) but had no effect on the mutant (GluR1S845A). (C) Quantitation of BBS-GFP-GluR1 plasma membrane insertion. Forskolin treatment increased both the cluster intensity (Left) and cluster size (Right) of the newly inserted surface BBS-GFP-GluR1 (n = 30; P < 0.05, t test).
Fig. 5.
A quick switch of GluR1S845 from a phosphorylated to a nonphosphorylated state is crucial to NMDA-induced AMPA receptor internalization. (A and B) NMDA treatment induces cell-surface GluR1S845 dephosphorylation. Plasma membrane AMPA receptors were isolated by using surface biotinylation and probed by using anti-phospho-GluR1S845 (GluR1 P-S845) antibodies. When receptor internalization was blocked with hypertonic sucrose solution (Sucrose), NMDA still caused a dramatic dephosphorylation (NMDA+Sucrose) of GluR1 (n = 2). (C) GluR1 is dephosphorylated at a rate faster than AMPA receptors are internalized after NMDA application. At 10 min after NMDA treatment, a large amount of AMPA receptors remained on the surface, but almost all of the surface receptors were dephosphorylated. (D) PKA preactivation enhances NMDA-induced AMPA receptor internalization. Cells were surface-biotinylated, incubated with forskolin for 5 min, and treated with NMDA. Endocytosed receptors were collected after surface stripping. PKA pretreatment increased the NMDA-induced AMPA receptor internalization (Pre-forsk), an effect that was blocked by H89 (Pre-forsk+H89). (E) Quantification of D. NMDA-caused AMPAR internalization was increased significantly by preforskolin treatment (n = 3; ∗, P < 0.05, t test).
Fig. 6.
Preventing dynamic GluR1S845 dephosphorylation abolishes NMDA-mediated GluR1 internalization. (A) Surface expression of GluR1-GFP in cortical neurons. Cultured cortical neurons transfected with GluR1-GFP or GluR1S845A-GFP (green) were immunostained with anti-GFP antibodies (red) under nonpermeant conditions. (B) NMDA fails to induce GluR1S845-GFP internalization. Surface receptors were labeled with antibodies against GFP, and receptor endocytosis was induced by using NMDA treatment. The internalized receptors were visualized after surface acid stripping. Note that NMDA treatment enhanced internalization of wild-type GluR1-GFP but had no effect on GluR1S845A. (C) Stripping immediately after surface biotinylation showed complete removal of surface labeling. (D) Quantitation of the internalization assays in B. NMDA treatment significantly increased GluR1-GFP internalization (n = 22; ∗, P < 0.05, _t_ test) but had no effect on the internalization of GluR1S845A-GFP (_n_ = 24; _P_ > 0.05, test). (E and F) In contrast, AMPA treatment (50 μM AMPA for 10 min) increased receptor internalization of both GluR1-GFP (n = 21; ∗, P < 0.05, t test) and GluR1S845A-GFP (n = 23; ∗, P < 0.05, t test).
Similar articles
- Enhancement of AMPA currents and GluR1 membrane expression through PKA-coupled adenosine A(2A) receptors.
Dias RB, Ribeiro JA, Sebastião AM. Dias RB, et al. Hippocampus. 2012 Feb;22(2):276-91. doi: 10.1002/hipo.20894. Epub 2010 Nov 15. Hippocampus. 2012. PMID: 21080412 - Activation of metabotropic glutamate receptors does not alter the phosphorylation state of GluR1 AMPA receptor subunit at serine 845 in perirhinal cortical neurons.
Harris SL, Gallyas F Jr, Molnar E. Harris SL, et al. Neurosci Lett. 2004 Nov 30;372(1-2):132-6. doi: 10.1016/j.neulet.2004.09.019. Neurosci Lett. 2004. PMID: 15531103 - Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors.
Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A. Essmann CL, et al. Nat Neurosci. 2008 Sep;11(9):1035-43. doi: 10.1038/nn.2171. Nat Neurosci. 2008. PMID: 19160501 - AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse.
Groc L, Choquet D. Groc L, et al. Cell Tissue Res. 2006 Nov;326(2):423-38. doi: 10.1007/s00441-006-0254-9. Epub 2006 Jul 18. Cell Tissue Res. 2006. PMID: 16847641 Review. - Auxiliary subunits assist AMPA-type glutamate receptors.
Nicoll RA, Tomita S, Bredt DS. Nicoll RA, et al. Science. 2006 Mar 3;311(5765):1253-6. doi: 10.1126/science.1123339. Science. 2006. PMID: 16513974 Review.
Cited by
- Homeostatic synaptic plasticity as a metaplasticity mechanism - a molecular and cellular perspective.
Li J, Park E, Zhong LR, Chen L. Li J, et al. Curr Opin Neurobiol. 2019 Feb;54:44-53. doi: 10.1016/j.conb.2018.08.010. Epub 2018 Sep 11. Curr Opin Neurobiol. 2019. PMID: 30212714 Free PMC article. Review. - Assembly of a beta2-adrenergic receptor--GluR1 signalling complex for localized cAMP signalling.
Joiner ML, Lisé MF, Yuen EY, Kam AY, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD, Burette AC, Weinberg RJ, Law PY, El-Husseini A, Yan Z, Hell JW. Joiner ML, et al. EMBO J. 2010 Jan 20;29(2):482-95. doi: 10.1038/emboj.2009.344. Epub 2009 Nov 26. EMBO J. 2010. PMID: 19942860 Free PMC article. - Adult medial habenula neurons require GDNF receptor GFRα1 for synaptic stability and function.
Fernández-Suárez D, Krapacher FA, Pietrajtis K, Andersson A, Kisiswa L, Carrier-Ruiz A, Diana MA, Ibáñez CF. Fernández-Suárez D, et al. PLoS Biol. 2021 Nov 8;19(11):e3001350. doi: 10.1371/journal.pbio.3001350. eCollection 2021 Nov. PLoS Biol. 2021. PMID: 34748545 Free PMC article. - Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation.
Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Lin DT, et al. Nat Neurosci. 2009 Jul;12(7):879-87. doi: 10.1038/nn.2351. Epub 2009 Jun 7. Nat Neurosci. 2009. PMID: 19503082 Free PMC article. - Long term synaptic depression that is associated with GluR1 dephosphorylation but not alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor internalization.
Davies KD, Goebel-Goody SM, Coultrap SJ, Browning MD. Davies KD, et al. J Biol Chem. 2008 Nov 28;283(48):33138-46. doi: 10.1074/jbc.M803431200. Epub 2008 Sep 26. J Biol Chem. 2008. PMID: 18819923 Free PMC article.
References
- Song I, Huganir RL. Trends Neurosci. 2002;25:578–588. - PubMed
- Malinow R, Malenka RC. Annu Rev Neurosci. 2002;25:103–126. - PubMed
- Sheng M, Hyoung Lee S. Neurosci Res (NY) 2003;46:127–134. - PubMed
- Bredt DS, Nicoll RA. Neuron. 2003;40:361–379. - PubMed
- Collingridge GL, Isaac JT, Wang YT. Nat Rev Neurosci. 2004;5:952–962. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources