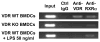Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: a paradigm for ligand-augmented negative transcriptional regulation - PubMed (original) (raw)
Review
Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: a paradigm for ligand-augmented negative transcriptional regulation
Matthew D Griffin et al. Arch Biochem Biophys. 2007.
Abstract
The immunological effects of vitamin D receptor (VDR) ligands include inhibition of dendritic cell (DC) maturation, suppression of T-helper type 1 (Th1) T-cell responses and facilitation of antigen-specific immune tolerance in vivo. While studying the molecular profile of DCs cultured in the presence of 1alpha,25(OH)D3 or synthetic D3 analogs we observed that expression of the NF-kappaB family member RelB, which plays an essential role in DC differentiation and maturation, is selectively suppressed by VDR ligands. Further in vitro and in vivo studies of VDR-mediated RelB suppression indicated that the mechanism for this effect involves direct binding of VDR/RXR alpha to a defined region of the relB promoter and assembly of a negative regulatory complex containing HDAC3, HDAC1, SMRT and, most likely, other factors. Interestingly, promoter engagement by VDR and HDAC3, but not the other identified components, is enhanced by addition of a VDR ligand and inhibited by a pro-maturational stimulus (LPS) that results in RelB upregulation. Promoter assays in a panel of cell lines suggest that the VDR ligand-dependent component of relB suppression may occur selectively in antigen presenting cells. Cell type-specific, ligand-enhanced negative transcriptional regulation represents a potentially novel paradigm for VDR-controlled genes. In this report we review the experimental data to support such a mechanism for relB regulation in DCs and present a model for the process.
Figures
Figure 1
Chromatin immunoprecipitation (ChIP) assay results are shown for day 7 bone marrow-derived DCs (BMDCs) from untreated VDR wild-type (WT) and VDR knockout (KO) mice and from VDR WT BMDCs treated overnight with 50 ng/ml LPS. Amplification of a 178 bp chromosomal DNA fragment incorporating the mouse relB promoter VDRE are shown for total genomic DNA and for protein/DNA complexes precipitated with Rabbit IgG (Control IgG) or with polyclonal rabbit antibodies against VDR (Anti-VDR) and RXRα (Anti-RXRα).
Figure 2
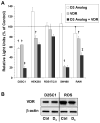
A. Results are shown for an experiment in which the effect of D3 analog treatment on transcriptional activity of a mouse osteopontin (mOPN) promoter construct was compared, by luciferase reporter assay, with that of a mouse relB promoter construct (mRelB) in an osteoblast cell line (ROS17/2.8) known to express VDR. Aliquots of transfected cells were treated with vehicle (Control) or with 10−10 M D3 analog. The results are expressed as mean ± SD luciferase activity from triplicate samples for each condition. † = p < 0.05 for D3 analog-treated vs. control-treated. B. The position and sequences are shown for the wild type VDRE within the mouse relB promoter and the mOPN VDRE generated within the relB promoter by site-directed mutagenesis. C. The effect of 10−10 M D3 analog treatment, VDR over-expression, or both on transcriptional activity of the wild-type mouse relB promoter was compared, by luciferase reporter assay in D2SC1 cells, with that of a mouse relB promoter construct in which the VDRE was mutated to the sequence of the mOPN promoter VDRE. The result is expressed as mean ± SD luciferase activity from triplicate samples for each condition. † = P < 0.05 compared to untreated control; ‡ = p < 0.05 compared to D3 analog alone and VDR alone.
Figure 3
A. The results of luciferase reporter assays in a panel of cell lines are shown. The effect of 10−10 M D3 analog treatment, VDR over-expression (VDR), or both (D3 Analog + VDR) on promoter activity of the wild-type mouse relB promoter was compared in the following cell lines: (i) D2SC1 (mouse DC), (ii) HEK293 (human embryonic kidney), (iii) ROS17/2.8 (rat osteoblast), (iv) SW480 (human colonic carcinoma), and (v) RAW (mouse macrophage). The results for each cell line are expressed as the % of untreated (control) cells for luciferase activity (mean ± SD of triplicate samples for each condition). † = P < 0.05 compared to untreated control; ‡ = p < 0.05 compared to D3 analog alone and VDR alone. B. Results of a Western blot for VDR and β-actin are shown for D2SC1 and ROS17.2.8 (ROS) cells following 24 hour culture in the absence (Ctrl) or presence (D3) of 10−7 M 1α,25(OH)2D3. Cultures were carried out using medium supplemented by charcoal adsorbed fetal calf serum to remove the potential influence of residual 1α,25(OH)2D3 in control conditions.
Figure 4
A. Schematic representations of a negative regulatory complex centered on the identified VDRE with the mouse relB promoter are shown for steady state (immature) DCs and DCs subject to a maturational stimulus. The components that have been shown experimentally to be constitutively or variably present by ChIP assay are VDR, RXRα, SMRT, HDAC1 and HDAC3. B. Schematic representations of two models for relB transcriptional suppression in DCs exposed to exogenous VDR ligand (1α,25(OH)2D3 or D3 analog).
Figure 4
A. Schematic representations of a negative regulatory complex centered on the identified VDRE with the mouse relB promoter are shown for steady state (immature) DCs and DCs subject to a maturational stimulus. The components that have been shown experimentally to be constitutively or variably present by ChIP assay are VDR, RXRα, SMRT, HDAC1 and HDAC3. B. Schematic representations of two models for relB transcriptional suppression in DCs exposed to exogenous VDR ligand (1α,25(OH)2D3 or D3 analog).
Similar articles
- Regulation of relB in dendritic cells by means of modulated association of vitamin D receptor and histone deacetylase 3 with the promoter.
Dong X, Lutz W, Schroeder TM, Bachman LA, Westendorf JJ, Kumar R, Griffin MD. Dong X, et al. Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):16007-12. doi: 10.1073/pnas.0506516102. Epub 2005 Oct 20. Proc Natl Acad Sci U S A. 2005. PMID: 16239345 Free PMC article. - Direct transcriptional regulation of RelB by 1alpha,25-dihydroxyvitamin D3 and its analogs: physiologic and therapeutic implications for dendritic cell function.
Dong X, Craig T, Xing N, Bachman LA, Paya CV, Weih F, McKean DJ, Kumar R, Griffin MD. Dong X, et al. J Biol Chem. 2003 Dec 5;278(49):49378-85. doi: 10.1074/jbc.M308448200. Epub 2003 Sep 24. J Biol Chem. 2003. PMID: 14507914 - Vitamin D-resistant rickets and type 1 diabetes in a child with compound heterozygous mutations of the vitamin D receptor (L263R and R391S): dissociated responses of the CYP-24 and rel-B promoters to 1,25-dihydroxyvitamin D3.
Nguyen M, d'Alesio A, Pascussi JM, Kumar R, Griffin MD, Dong X, Guillozo H, Rizk-Rabin M, Sinding C, Bougnères P, Jehan F, Garabédian M. Nguyen M, et al. J Bone Miner Res. 2006 Jun;21(6):886-94. doi: 10.1359/jbmr.060307. J Bone Miner Res. 2006. PMID: 16753019 - The vitamin D hormone and its nuclear receptor: molecular actions and disease states.
Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, Selznick SH, Whitfield GK. Haussler MR, et al. J Endocrinol. 1997 Sep;154 Suppl:S57-73. J Endocrinol. 1997. PMID: 9379138 Review. - Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands.
Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M. Adorini L, et al. J Steroid Biochem Mol Biol. 2004 May;89-90(1-5):437-41. doi: 10.1016/j.jsbmb.2004.03.013. J Steroid Biochem Mol Biol. 2004. PMID: 15225816 Review.
Cited by
- 1,25-Dihydroxyvitamin D3 promotes a sustained LPS-induced NF-κB-dependent expression of CD55 in human monocytic THP-1 cells.
Izban MG, Nowicki BJ, Nowicki S. Izban MG, et al. PLoS One. 2012;7(11):e49318. doi: 10.1371/journal.pone.0049318. Epub 2012 Nov 12. PLoS One. 2012. PMID: 23152895 Free PMC article. - 1α, 25 Dihydroxyvitamin D (1,25(OH)2D) inhibits the T cell suppressive function of myeloid derived suppressor cells (MDSC).
Fleet JC, Burcham GN, Calvert RD, Elzey BD, Ratliff TL. Fleet JC, et al. J Steroid Biochem Mol Biol. 2020 Apr;198:105557. doi: 10.1016/j.jsbmb.2019.105557. Epub 2019 Nov 26. J Steroid Biochem Mol Biol. 2020. PMID: 31783150 Free PMC article. - Role of Vitamin D in Head and Neck Cancer-Immune Function, Anti-Tumour Effect, and Its Impact on Patient Prognosis.
Starska-Kowarska K. Starska-Kowarska K. Nutrients. 2023 May 31;15(11):2592. doi: 10.3390/nu15112592. Nutrients. 2023. PMID: 37299554 Free PMC article. Review. - 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1.
Kovalenko PL, Zhang Z, Cui M, Clinton SK, Fleet JC. Kovalenko PL, et al. BMC Genomics. 2010 Jan 13;11:26. doi: 10.1186/1471-2164-11-26. BMC Genomics. 2010. PMID: 20070897 Free PMC article. - Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer.
Meeker S, Seamons A, Paik J, Treuting PM, Brabb T, Grady WM, Maggio-Price L. Meeker S, et al. Cancer Res. 2014 Aug 15;74(16):4398-408. doi: 10.1158/0008-5472.CAN-13-2820. Epub 2014 Jun 17. Cancer Res. 2014. PMID: 24938764 Free PMC article.
References
- Adorini L. Curr Opin Invest Drugs. 2002;3:1458–63. - PubMed
- Deluca HF, Cantorna MT. FASEB Journal. 2001;15:2579–85. - PubMed
- Griffin MD, Kumar R. J Steroid Biochem Mol Biol. 2005;97:213–8. - PubMed
- Hayes CE, Nashold FE, Spach KM, Pedersen LB. Cell Mol Biol. 2003;49:277–300. - PubMed
- Mathieu C, Adorini L. Trends Mol Med. 2002;8:174–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 DK073369/DK/NIDDK NIH HHS/United States
- R21 DK077669/DK/NIDDK NIH HHS/United States
- R01 DK25409/DK/NIDDK NIH HHS/United States
- R01 DK059505/DK/NIDDK NIH HHS/United States
- R01 DK058546/DK/NIDDK NIH HHS/United States
- R01 DK65830/DK/NIDDK NIH HHS/United States
- R01 DK58546/DK/NIDDK NIH HHS/United States
- R01 DK076829/DK/NIDDK NIH HHS/United States
- R01 DK025409/DK/NIDDK NIH HHS/United States
- R01 DK59505/DK/NIDDK NIH HHS/United States
- R01 DK065830/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous